Wireless data collected directly from patients during normal physical activities could enable the development of better knee implants
March 22, 2007
Originally Published MPMN March 2007
PROFILE
Sensors Measure 3-D Force and Torque Data in Human Knee Replacement
Wireless data collected directly from patients during normal physical activities could enable the development of better knee implants
|
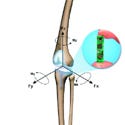
Wireless transmitter electronics integrated into a knee implant report digital 3-D torque and force data. |
Historically, knee implants have been designed using predictions based on theoretical data. Now, for the first time, a patient has received an investigational full artificial knee replacement that can wirelessly report digital 3-D torque and force data. With this new technology, the smart total knee replacement can transmit multiaxis loading information directly from the patient to a computer.
The latest iteration of the implant represents a significant technological advancement from the original version, implanted in 2004, which only reported knee compressive forces. In addition to compressive forces, the second-generation smart implant reports on twisting, bending, and shearing loads across the human knee. Forces and torques transmitted across the knee joint during such normal activities as stair climbing, rising from a chair, and walking provide input that researchers can apply to improving implant design, refining surgical instrumentation, guiding postoperative physical therapy, and detecting the individual activities that could overload the implant.
Development of the secondgeneration implant proved to be a collaborative effort between several clinicians, scientists, and manufacturers dating back more than a decade. Building on the original version, the Scripps Clinic (San Diego; www.scripps.org), under the direction of Darryl D’Lima and Clifford Colwell, required robust, yet small electronics to facilitate expanded reporting capabilities.
Researchers had employed telemetry to measure forces in the hip, spine, and femur, but the available space in a knee replacement had posed severe barriers in the past. Owing to their microminiature size and micropower, wireless transmitter electronics and multichannel strain-measurement components provided by MicroStrain Inc. (Williston, VT; www.microstrain.com) enabled a breakthough.
A custom titanium alloy total knee replacement serves as the basis for the device. Designed with a hollow stem portion, a custom-designed tibial component houses MicroStrain’s wireless strain-gauge electronics. After the array of 12 sensitive piezoresistive strain gauges is embedded within the tibial component, a polyethylene cap is threaded onto the distal end of the stem to protect the hermetically sealed radio antenna. The finished implant is then hermetically sealed using laser welding techniques and is tested for hermeticity using fine helium leak detection methods.
Batteries are not needed to power the device. Instead, a miniature coil located within the implant harvests energy from an externally applied alternating field. The remote powering coil is secured to the outside of the patient’s shin, away from the knee.
Using a wireless antenna, the implant transmits digital sensor data from the 12 strain gauges to a computer in a readable format. The computer then uses a stored calibration matrix to convert the raw strain data about the knee into 3-D torque and force measurements. The second-generation implant handles 12 channels of strain data, as compared with only four strain-gauge channels for the original system.
For the first time, orthopedic researchers have the tools to measure 3-D forces and torques in live human knees. As the recipient of this smart implant progresses during rehabilitation, 3-D load and torque data will be collected for the first time during such activities of daily living as walking, climbing stairs, and running. The system is currently a research device and is not yet commercially available.
Copyright ©2007 Medical Product Manufacturing News
You May Also Like