SOFTWARE
|
All medical device manufacturers share a common goal: quality. By relentlessly pursuing this goal, device manufacturers seek to satisfy existing customers and win new ones, while avoiding the costly and reputation-sullying consequences of repeated field failures and product recalls.
Many times, though, the pursuit of quality is hindered by cumbersome paper-based recordkeeping systems used to track production activity. These paper systems are subject to human errors that can result in production delays, or worse, shipment of faulty products. They also make it difficult for manufacturing personnel to reliably trace components and extract critical data needed to improve the process, reducing the efficiency of production and hampering efforts to improve product quality. As companies grow, paper-based systems become more unwieldy and problematic, eventually spurring many manufacturers to look for an alternative.
Seeking improved compliance, quality, and efficiency, growing medical device manufacturers are replacing their paper records with automated systems featuring manufacturing execution system (MES) software. These software-based systems enforce adherence to correct manufacturing procedures and eliminate both recordkeeping errors and the time-consuming reviews meant to catch them. They also improve component traceability and allow quick and easy access to key product and process data. Other benefits include increased line yield and throughput, as well as significantly reduced cycle time and out-of-box failures.
|
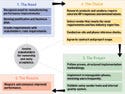
Figure 1. (click to enlarge) These four steps can help companies choose and deploy the right MES. |
Before realizing these benefits, a company must travel the road to successful MES operation. Key steps along this road include choosing the right MES product and implementing the system at the company. These steps significantly affect the gains that come from computerized recordkeeping (see Figure 1).
Paper-Based Problems
During production, regulated medical device manufacturers document compliance by keeping paper device history records (DHRs). Filled out by operators along the production line, DHRs can be compromised by omissions, illegible entries, and errors such as values that are outside of specification limits. For those reasons, firms employ a group of inspectors to check all the DHRs at the end of the process. When these inspectors find omissions or errors in the paperwork, they go back to the operators to try to get the necessary information.
Besides being inefficient, a paper-based recordkeeping system adds risk. For example, a process can continue to turn out flawed products until an operator catches a measurement value that is out of spec. In addition, an omission or illegible DHR entry might require a product to be retested so that the missing value can be obtained. Then there's always the possibility that DHR mistakes will go undetected.
Paper records can affect product quality in other ways as well. With vast quantities of production data stored in numerous file cabinets on the shop floor and elsewhere, tracing problems back to a particular component or process is difficult and time-consuming. And because recordkeeping requirements add significant overhead, some medical device manufacturers are reluctant or unable to collect and analyze additional data that would help them improve quality. Obviously, rapid growth only exacerbates the issue.
A Better Way
Rather than just tinkering with a flawed process, medical device firms can phase out their paper-based recordkeeping systems and replace them with computerized systems run by an MES. Numerous warning letters and consent decrees have their origin in manufacturing paperwork.
An MES automatically enforces adherence to the manufacturing process, ensuring that all tasks are completed (with an electronic signature, when deemed necessary by the manufacturer), done in the proper sequence, and performed by properly trained operators.
In addition, an MES ensures that all process data are collected (eliminating paper-related problems such as omissions and illegible entries) and that measurements and test results meet specifications. It also detects nonconformances and ensures that production problems trigger appropriate corrective actions.
In addition to its key role in the production process, an MES replaces scattered file cabinets with a computerized database. This makes it easy for users to trace components and find important product and process information. It also provides a secure backup of these important data files.
An MES works in tandem with quality software modules supplied by partners of the MES vendor so that the MES is a complete and integrated system for tracking quality issues. Modules include those for corrective and preventive action and software that handles customer complaints. An MES is also integrated with core business systems such as an enterprise resource planning system to provide accurate data for planning and accounting.
Selling the Idea
Like any other important change at a company, the switch to an MES will have to be justified to corporate executives.
Companies should estimate the costs of software licensing; hardware and infrastructure; team education; professional services to assist with project management, modeling and configuration; system validation; end-user training; and the overhead of temporary parallel processes. In addition, ongoing costs such as software maintenance and upgrades should be included.
In addition to a carefully prepared estimate of how much the switch will cost, a proposal to the CEO or management team should include an explanation of how a software-based manufacturing system can help prevent disasters that can seriously damage the company's reputation, eliminate costly non-value-added activities, and increase revenue.
For example, an MES can prevent repeated field failures by making it easy for engineers to find information that helps them solve a problem caused by a particular component. In addition, the software can help plant personnel trace production problems to a particular machine at a certain time of day. Such information might limit the scope of a recall to a handful of products rather than thousands.
The financial benefits of shortened cycle times, improved yields, decreased production downtime, and reduced nonconformances can also be provided to support implementing an MES. Manufacturers can calculate the hard costs of carrying excess inventory, of scrap and rework, and of addressing nonconformances.
In addition, growing companies that are introducing new products and those that can sell everything they can produce will benefit from the top line: Increased revenue is a direct result of delivering good product to the customer as quickly as possible. It is wise to calculate how the benefits continue year after year, increasing the value over time.
MES advocates must get the support of the company's IT steering committee, the group that decides which of the competing IT projects under consideration are most important. The proposal to this group should include the benefits and costs of an MES, as well as the technology and staff requirements for implementation.
Choosing a System
Once corporate management signs off on the switch to an MES, the next step is choosing a software package. A good selection process starts with the creation of a cross-functional team tasked with developing business requirements that will be used to evaluate alternatives. These requirements will be matched up with specific features of the products under consideration. Specific requirements are more helpful in the selection process than general ones, which may be met to some degree by all the contenders, making it difficult to choose between them.
Ideally, the team tasked with developing business requirements will be able to focus exclusively on the job for a certain period of time. A team that goes off-site for a week for the sole purpose of producing a list of business requirements will probably do a much better job than a team that must develop requirements during a series of one-hour meetings held over a period of days or weeks.
Once developed, business requirements shouldn't be carved in stone. The team should be open to adding requirements in response to information acquired during the selection process. While viewing product demonstrations, for example, the team may discover that a couple of the competing software packages meet a corporate need that no one thought could be met by the products under consideration. When the team learns that a need can be met, it should be added to the business requirements used to evaluate the products.
Demos of the competing MES products should be viewed by a large group from all areas of the company. Besides providing diverse input that will improve the selection process, the members of this group will get information about MESs that will be useful when the selected system is installed. For example, manufacturing routing concepts such as workflow will likely be explained, and personnel can see how work flow can be configured using an MES.
When evaluating products, the group should focus on key attributes such as out-of-the-box functionality, which is what the software is designed to offer without custom coding by the user. Examples of out-of-the-box functionality include end-user configuration of work flows and specifications, integrated nonconformance management, electronic signatures, user interfaces that direct operators and minimize data entry, and reports that are easy to read and configure. If an application requires custom code for such features, it will probably rank low in out-of-the-box functionality.
An MES application will need to be validated for its intended use, i.e., in the manufacturing plant. First, the software as delivered must be fully compliant with Title 21 CFR Part 11. Next, the vendor should supply tools that will accelerate validation. These tools include the documentation, procedures, and validation protocols that help ensure that the quality systems and production processes leveraging the MES are operating efficiently and comply with the latest FDA guidelines and regulations.
If a data field includes upper and lower limits that can be set by users without custom coding, the field is said to be configurable. In general, people evaluating MES software should look for a system with many configurable features rather than one that requires coding to customize it for a particular application.
Besides sizing up the different MES products, the selection process should include an assessment of the software vendors. Key questions to answer about the vendors include:
• Are they solid companies with plenty of experience in developing and supporting MES software?
• Will their people be helpful and easy to work with? You may be depending on them for the next 5–10 years.
• How much experience do they have with regulated manufacturing firms? Software vendors with many life sciences customers should be happy to provide that information. Those with little or no medical device experience may be evasive or refuse to answer the question.
• Is their software designed, developed, and released in a controlled process? The vendor should have recent, satisfactory audit results.
• What long-term help can you get from their customers? Some vendors offer forums to communicate with other customers that can offer helpful advice on subjects such as how to train people to use MES software, how to phase in implementation, and when and where to use electronic signatures.
Of course, it's also a good idea to interview and visit vendors' references for information about each MES alternative and their experiences with the vendors. Feedback from other medical device manufacturers is essential.
When all the selection information is in, it's time to make a choice. In all probability, each system will have both strong and weak points. So it may be helpful to use a scoring system to rate the options. Scores can be based on how each option measures up to the business requirements, ranked in order of importance.
Implementing the New System
With the selection process concluded, the equally important process of MES implementation begins. In most cases, implementation takes place in phases. The implementation team should decide how to phase in an MES. Like the team that developed the business requirements, the implementation team should be a cross-functional group that includes representatives of all key departments of the company, including IT, manufacturing, and quality. The team should also include project managers from the manufacturer and the software vendor.
Typically, companies start by implementing features that will meet the minimum requirements for producing completely electronic DHRs. These features establish a manufacturing compliance platform and mitigate compliance risks. The second phase can include additional data collection and controls to reduce variability and increase throughput. The third phase adds the analysis of this new breadth and depth of data to continually improve processes.
A concurrent approach is to implement each phase by product line. Most companies start with a challenging product line for which streamlined processes will provide the greatest benefit. Examples include a product whose manufacturing processes or bill of material are complex and difficult to track on paper, or a product whose volume is increasing and would be slowed by continuing to use paper, or a product whose yields or field failures are not at desired levels.
One of the most important—and most difficult�—tasks for the implementation team is educating staff about the MES. The capabilities of manufacturing software can be hard to grasp for people used to conventional recordkeeping tools. But learning will eventually lead to buy-in as people begin to understand what an MES can do for them.
The implementation team can also boost buy-in by getting input from users on how the software should be configured. For example, the team can ask operators to test a user interface and suggest ways to improve it. Or the team can ask engineers to critique an MES report format. By soliciting and acting on this type of feedback, the team gives company personnel an ownership stake in the MES project. Buy-in aside, this feedback is valuable because it helps the team make the system easier to use.
Validation will be required, and the implementation team must plan for it. The plan will include the scope, assumptions, roles and responsibilities, and acceptance criteria. Thorough requirements must be documented, including user requirements and functional requirements. These are the baselines for the traceability matrices used in the software qualification test protocols: installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ).
Regulated companies should operate the electronic system in parallel with the old system for a limited time (PQ), to prove that its results are equal to (and often better than) the old system's results. If end-users have been involved throughout the implementation, the duration of parallel processing is often short, because issues have already been exposed and resolved.
In any case, it is advisable to use a risk-based approach, in which the validation process is thorough and verifiable, but does not overburden the effort with excessive interpretation of the regulations.
Among companies switching to computerized data-collection systems, a common concern is what happens if the system goes down. Many companies can't afford manufacturing downtime caused by an MES malfunction. Understandably, however, these companies don't want to back up their electronic recordkeeping system with an extensive paper-based system like the one they're replacing.
So the implementation process should include the installation of redundant systems that ensure 100% uptime and data integrity. That is, if one system fails, there can be a seamless switchover to the other without any production downtime.
MES implementation should also include the installation of a plantwide bar coding system. Bar coding boosts the speed and accuracy of data entry by eliminating manually keyed data entry. Ideally, all product components would be bar coded. At a minimum, though, suppliers or plant personnel should bar code expensive components and those that can cause product failure. Bar coding ensures that the serial numbers of these key components are correctly entered into the system, so they'll be easy to trace if necessary.
Results
When an MES is up and running, medical device manufacturers will notice many welcome changes on the plant floor and beyond. Of course, one of these changes is a dramatic reduction in shop-floor paperwork and file cabinets. Another is the reduction of labor-intensive DHR reviews. In some cases, end-of-process DHR reviews can be completely eliminated. QA inspectors are now free to help the company improve processes and quality.
One of the most important features of an MES is its ability to detect and react to production problems early in the process. If a product or process value is outside of specification limits, for example, an MES detects it and automatically takes one or more prescribed actions. These actions could include directing the product to a rework station, sending a message to engineering, and generating a nonconformance report that describes the problem and the specific steps taken to correct it.
|
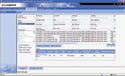
Figure 2. (click to enlarge) A screen shot shows a typical electronic manufacturing audit trail. |
An MES also provides quick and easy access to product and process data. The system centralizes all data in a searchable form, including both shop-floor data and product performance information from customers and service centers. Therefore, an MES can provide users with a complete manufacturing audit trail of a product (see Figure 2).
An MES database provides valuable process data not normally or easily extracted from paper-based systems. For example, an MES can help plant personnel uncover rework loops that reduce manufacturing efficiency and increase the risk of product failure. Tracking rework in paper-based systems requires looking through paper DHRs to piece together what happened. Some paper recordkeeping systems don't even require operators to report that rework was done on a product, only that the product was good when it left their station.
The hunt for rework is easier with an MES, which can be set up to require the recording of all rework done by an operator. Such recording helps manufacturers zero in on the processes that require the most rework and therefore need improvement.
An MES also makes it easy to trace components and assess their condition and performance. This is difficult when data collection is done using a paper-based system. A manufacturer seeking information about a component in a particular type of device would have to retrieve the paper DHRs, as well as any repair data on file at service centers that might be scattered across the country or even worldwide.
By contrast, a manufacturer can quickly retrieve all of the DHR and repair data for a device simply by querying an MES-based data-collection system. These data can be used to quickly trace failures back to components made by particular vendors. For example, a query to the system might show that nine of the last 10 valves that failed in the field were made by Vendor A. This would tell the manufacturer to focus on Vendor A rather than taking the matter up with all of its valve suppliers.
The query might also retrieve additional information, such as what went wrong with the valves and whether they were all from the same lot. This information can be supplied to Vendor A to help the supplier solve its quality problem.
With this type of component data, manufacturers can create scorecards that help them monitor and compare the performance of different suppliers. These scorecards can provide useful information such as the mean time to failure (MTTF) for each supplier's products, whether and how much each product's MTTF is increasing over time, and which supplier's products have improved the most in a certain time period.
As a key component of the manufacturing process, an MES yields many other benefits, including:
• Less work in progress (WIP) inventory. Every lot, batch, or unit is tracked through every operation. Improved visibility of WIP helps plant personnel identify and eliminate production bottlenecks.
• Increased line yield. Early detection of problems, easier access to data leading to their cause, and resulting process and training improvements can increase yield more than 10%.
• Increased throughput and reduced cycle time. Minimizing non-value-added activities such as paperwork, rework, and redundant checks can boost throughput by almost 40% and slash cycle time by nearly 25%.
• Fewer nonconformances. Process improvements made possible by easier and greater access to product and process data can cut nonconformances nearly in half.
• Fewer out-of-box failures. MES-related process improvements can also reduce field failures by almost 70%.
• Lower risk. In-process controls ensure that employees have the required training before working an operation, thereby reducing opportunity for errors.
Conclusion
Medical device manufacturers can reap many benefits by switching from paper recordkeeping systems to automated data-collection systems based on MES software. The process begins by cataloging the drawbacks of paper-based systems and the potential improvements that can be gained from a software-based alternative. This information can be used to justify the new system to corporate decision makers.
The best course of action is to develop and follow a detailed plan for choosing an MES product and then implementing the chosen system in a way that meets the needs of the company and its personnel. An MES enables medical device manufacturers to effectively manage rapid growth while improving both their production processes and the quality of their products.
Jason Knight is senior manager of quality engineering at Kinetic Concepts Inc. (San Antonio, TX). Susan Lamb is senior manager of product marketing at Camstar Systems Inc. (Charlotte, NC) and can be reached at [email protected].
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like