CDRH's increased emphasis on postmarket device safety is driving the development of a new eMDR system.
July 1, 2007
INFORMATION TECHNOLOGIES
Sidebar: Complaint Handling and Medical Device Reporting Software Vendors |
In 2006, FDA launched what the agency refers to as a postmarket transformation initiative. Developed through FDA's Center for Devices and Radiological Health (CDRH), the initiative is designed to enhance postmarket monitoring of medical devices, enabling FDA to identify, analyze, and act on problems more quickly.
According to leaders of the ongoing initiative, "CDRH is committed to achieving a seamless approach to the regulation of medical devices. In such an environment, the center's premarket evaluation activities would be integrated with continued postmarket vigilance and enforcement, and appropriate and timely information would be fed back to all of CDRH's stakeholders."1 Examples of CDRH stakeholders include healthcare practitioners, patients, manufacturers, and the public at large.
|
Photo by iStock |
CDRH refers to such an integrated concept as its total product life cycle model, a philosophy through which the center strives to meet the following complementary goals.
Getting safe and effective devices to market as quickly as possible.
Ensuring that devices currently on the market remain safe and effective.
Prior to the initiative's launch, CDRH had focused much of its efforts on the first of the two goals. But now, in the wake of multiple high-profile medical device recalls—and despite the fact that the agency has had programs for ensuring the long-term safety of devices in place for more than 20 years—renewed emphasis is being placed on the postmarket stages of the total product life cycle (see Figure 1).
Postmarket surveillance becomes important the moment a medical device is released onto the market, and such surveillance remains crucial throughout the product's life cycle. The following are among FDA's reasons for its increased emphasis on postmarket monitoring.
Increased technological risk: Devices are becoming smaller, smarter, and more complex.
Increased risk of failure: Simply stated, patients are outliving the life expectancy of their devices; the need to evaluate the reliability and durability of devices is becoming more apparent.
Disjointed information about safety issues: Postmarket information is difficult to analyze because it comes from many sources. Furthermore, such information may be missing, incomplete, inaccurate, inaccessible, or lacking timeliness.
Increases in device recalls and reports of adverse events (see Figure 2).2
Information Management's Critical Role
CDRH's postmarket surveillance efforts are focused on gathering, assessing, and responding to safety threats that arise once a device is in use. Thus, information management is critical to the success of the postmarket transformation initiative. CDRH recognized the importance of well-managed information back in 1999, when the agency outlined the need for the Medical Device Surveillance Network (MedSun). According to CDRH, the surveillance system—now referred to as the Medical Product Safety Network—would need to achieve the following objectives in order to accomplish its goal of increasing and sustaining the safety and effectiveness of medical devices.
Collect high-quality data about adverse medical device events.
Analyze the data to identify newly emerging device problems and changes in device use.
Disseminate data regarding newly emerging device problems in a timely manner to concerned parties, especially healthcare professionals and the public.
Apply the knowledge gained from the reported data to the device approval process and to prevention and control programs focused on patient safety.
Provide the findings regarding emerging device problems to medical device companies to aid them in making appropriate changes to design controls and human-factors issues.3
|
Figure 1. The total product life cycle spans all activities from a device's development to its ultimate disposal. |
In many ways, FDA's current information-gathering effort is akin to pointing a radio telescope into deep space, searching for faint signals of unusual activity. CDRH relies on several different postmarket sources of information to identify issues, patterns, and trends in medical device safety throughout the universe of the healthcare system. These sources include the following.
MedSun. Initiated as a pilot program in 2002, MedSun enables user facilities to report problems with devices via the Internet. To date, CDRH has enrolled 350 healthcare facilities in the network.3
Postapproval Studies. Occasionally, FDA approves a medical device before all long-term questions about its safety and effectiveness have been answered. If these questions can only be answered by a large clinical study, FDA may approve the device for sale but require the manufacturer to continue to study its safety and effectiveness. This effort typically applies to Class II or Class III devices that present higher degrees of risk due to their role in sustaining or supporting life.
|
Figure 2. Medical device reports received by CDRH, 2000-2006. |
Complaints. All medical device manufacturers are subject to the complaint management requirements in FDA's quality system regulation (QSR).4 A complaint, which may be lodged against any finished device released for distribution, is defined as an indication that a device has failed to meet customer or user expectations for quality or to meet performance specifications.
Facility Inspections. Under federal law, FDA has the authority to conduct good manufacturing practices inspections of medical device manufacturing facilities. During these inspections, FDA has the authority to require a device firm to open its complaint files, and review and copy documents from the files. In addition, facilities, manufacturing processes, records, and corrective action programs may be examined by FDA investigators. The results of an inspection provide the information necessary to evaluate a manufacturer's compliance with the QSR, as well as medical device reporting requirements.5
Adverse-Event Reporting. Anyone, including consumers, can file an adverse-event report. Voluntary reports can be filed under CDRH's MedWatch program. Medical device reports (MDRs)—mandatory in the case of death, serious injury, or major malfunction—are the mechanism by which FDA receives information about significant medical device adverse events from manufacturers, importers, and user facilities.
Digging Deeper into MDRs
MDRs are the most widely used mechanism by which manufacturers, importers, and user facilities, such as hospitals, report adverse events. Voluntary reports may be filed by healthcare professionals and consumers when an adverse event is noted spontaneously in the course of clinical care.
Manufacturers, importers, distributors, and user facilities must file mandatory MDRs with CDRH when a device may have caused or contributed to a death or serious injury, or when a similar device malfunction would likely cause, or contribute to, a death or serious injury were the malfunction to reoccur.
|
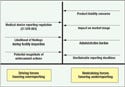
Figure 3. Medical device manufacturers face a number of opposing forces when deciding when to submit medical device reports (MDRs) to FDA's Center for Devices and Radiological Health. Each company's approach to MDRs will be dictated by the stance that management takes toward these opposing forces. In this diagram of a force field analysis, the compelling reasons to file MDRs are shown as driving forces, while erroneous reasons for not filing are shown as restraining forces. A major issue underlying the restraining forces is that an MDR is a matter of public record, available to potential customers, competitors, and consumers. |
In 2006, nearly 220,000 MDRs were filed—an average of almost 20,000 per month, or about 1000 per business day.2 Despite this large volume, adverse events are believed to be significantly underreported for a number of reasons. Moreover, when they are filed, their submission is often beyond the deadlines mandated by MDR requirements (see Figure 3).
FDA's database of warning letters is testament to the number of manufacturers that have, at one time or another, assumed the risks of postmarket reporting noncompliance. In its warning letters, FDA frequently cites a company's lack of a formal process for reviewing, evaluating, and investigating complaints, and reporting the serious cases to the agency via the MDR system. Deficiencies usually stem from improper complaint handling, as every complaint must be evaluated to determine whether it is reportable.
The Promise of eMDRs
In November 2006, CDRH's postmarket transformation leadership team issued a report that discussed shortcomings of the MDR system's current methods of receipt, processing, and analysis. In its report, the team stated,
The center's goal is to develop the ability to collect data on postmarket device performance from both regulatory and nonregulatory sources and be able to efficiently analyze that data to detect signals of adverse device performance. Currently, the main source of information on postmarket device performance is derived from MDR reports . . . Over a dozen years of history with this system has revealed that device-related adverse events are vastly underreported and that the data that are reported are often incomplete and unreliable.1
In its report, the team recommended that CDRH introduce electronic reporting not only for MDRs from manufacturers, but also for adverse-event reports from MedSun member institutions. Furthermore, the authors of the report stated that electronic submission of medical device reports (eMDRs) should be mandated, not just encouraged. Proponents of the shift to electronic reporting say that such a move will reduce submitters' administrative burden, lower their cost of compliance, help them become more timely in meeting submission deadlines, and reduce the number of supplemental reports they have to file.
Building an eMDR System
The infrastructure for moving medical device reporting to an electronic format has been in place for years. FDA's existing Electronic Submissions Gateway provides a centralized, agencywide switchboard for receiving electronic regulatory submissions securely. The system can be likened to that of an old-fashioned telephone switchboard, where an operator connects callers with the party who they are trying to reach. Fortunately, today's technology enables each submission to be automatically and securely routed to its correct recipient without the need for staff intervention. The Electronic Submissions Gateway identifies the type of submission received, the type of organization that submitted it, and to which of FDA's centers it should be routed.6
In addition to an infrastructure, electronic data exchange such as that of an eMDR requires a common language in which systems can communicate. Fortunately, such languages are also already in place. Extensible markup language (XML), which has been widely adopted for the purpose of exchanging data and documents across the Internet, gives consistent structure, meaning, format, context, and grammar to data.
A more sophisticated information exchange standard called Health Level 7 (HL7) is also becoming available. As a standards-writing body, HL7's primary mission is to enable the exchange and interoperability of patients' electronic health records. Such records are sophisticated documents that may contain text, data, electrocardiogram strip-charts, x-ray and ultrasound images, and possibly even video in the future. However, although HL7 and XML each serve a purpose, XML has been more widely adopted for most situations because of its simplicity and flexibility.
In addition to the infrastructure backbone of the Electronic Submissions Gateway and common information languages, CDRH already has some experience in digitizing the MDR process. An example is the MedWatch system for voluntary reporting of adverse experiences. MedWatch provides consumers, practitioners, and other members of the healthcare community with an easy way to report disconcerting or dangerous experiences with medical devices, drugs, and related products. Such experiences can include unexpected reactions, product quality issues, and use errors that range from annoying to alarming. The MedWatch program is based on the idea that everyone should have the opportunity to report adverse experiences to FDA.
For years, the agency has provided access to a hard copy of the voluntary adverse-event reporting form—FDA Form 3500—via the MedWatch portion of its Web site. Submitters could access the form through the site, fill it in, and send it to FDA via traditional methods, such as mail, fax, or e-mail. A couple of years ago, FDA took this process a step further and created a Web-based version of the form, which enables users to submit their voluntary reports online through a portal that has the look and feel of one used for online shopping.7
Emerging eMDRs
Over the past year, CDRH has been working to extend electronic reporting capabilities to the mandatory MDR system. Manufacturers, importers, distributors, and user facilities file these reports on FDA Form 3500A, a hard copy version of which can be downloaded from the agency's Web site.
In May 2007, CDRH announced the availability of its new eMDR capability. The new option is designed as an alternative to the current paper-based system, which requires FDA staff to spend considerable resources reentering data into the adverse-events system from reports received through mail, faxes, e-mails, and phone calls.
According to CDRH, the new eMDR system will reduce the reporting burden for both large- and small-volume medical device adverse-event reporters, and will improve the center's ability to detect postmarket medical device issues.
The new system gives manufacturers the ability to submit their medical device reports via one of two electronic pathways, both of which require submitters to register with the Electronic Submissions Gateway. Large manufacturers, which potentially submit hundreds of reports a year, can use a batch submission protocol that makes use of standards like XML and HL7. Because FDA's use of HL7 is relatively new, the standard is not yet addressed on the Web site of the Electronic Submissions Gateway. Thus, manufacturers should contact FDA directly to ensure their systems are compatible with the submission protocol.
On the other hand, small manufacturers with a limited number of reports can use a vehicle known as CDRH eSubmitter (CeSub) by downloading special software from CDRH's Web site.8 The software runs on a personal computer and enables the submitter to enter one report at a time on a computer-based version of the 3500A.
CeSub evolved as a result of two CDRH pilot programs, Turbo 510(k) and eLaser. Turbo 510(k) enables manufacturers to electronically complete and submit premarket notification applications to the Office of In Vitro Diagnostic Device Evaluation and Safety. Meanwhile, eLaser enables companies to electronically complete and submit a variety of product reports for radiation-emitting products to the Radiological Health Program. And, as of May 2007, CeSub includes the ability to create a 3500A form for mandatory medical device reporting.
The MDR functionality is so new to CeSub that, as of press time, the system's online user guide did not yet mention the new capability. In addition, the system has yet to enable manufacturers to report their MDRs through a Web portal. Rather, submitters using CeSub use a personal computer to collect the required information, compile a medical device report, convert it to a PDF format, and then burn a CD of the documents. The CD is then sent to CDRH. The time savings occur mainly on the agency's side. Receiving reports in an electronic format eliminates the need for CDRH staff to reenter data into the agency's system.
As CDRH continues to enhance the system, CeSub will migrate toward a Web-based method of submission. Pilot programs using such technology are underway at other centers within FDA. And although CDRH has been behind other FDA groups in adopting electronic submission technology, it doesn't take much imagination to see the day-to-day usefulness of a Web-based eMDR system for small-volume submitters.
In the meantime, the emergence of electronic reporting for mandatory MDRs has caught the attention of software vendors that specialize in the medical device industry. Both Sparta and EtQ have been working with FDA to develop functionality within their software that enables manufacturers to compile relevant product complaint and adverse-events data and electronically transmit MDRs through the Electronic Submissions Gateway. Other software vendors like Pilgrim, AssurX, and MasterControl are likely to have their eye on developing similar functionality. MDR reportability decisions and the process of submitting MDRs are tightly coupled with the complaint-handling process. Any device manufacturer that already has an automated complaint-handling system would be well-served to check with its current complaint-handling software vendor regarding its plans to support eMDRs. If a manufacturer is still thinking of evaluating software in the area of complaint handling, it would do well to include eMDRs in its list of requirements.
Managing Change
The emergence of eMDRs moves CDRH's postmarket transformation initiative forward in several respects. By eliminating the need for the center's staff to reenter MDR data into the adverse-events reporting system, the center frees up valuable agency resources—which are already in short supply—and reduces the potential for data entry errors on the behalf of both submitters and CDRH staff. While the system—still very much in its test phase—has yet to become the mandatory submission method endorsed by CDRH's postmarket transformation leadership team, it is likely that more and more MDRs will be submitted via this electronic pathway in the future.
No matter which protocol a company uses—paper, batch, CD, or Web—postmarket compliance begins with well-designed and documented policies and procedures, people who are trained and motivated to adhere to them, and evidence that such policies and procedures were followed. New tools and technologies to make this process more efficient are rapidly becoming available.
References
1. Report of the Postmarket Transformation Leadership Team: Strengthening FDA's Program for Medical Devices (Rockville, MD: Center for Devices and Radiological Health, FDA, 2006); available from Internet: www.fda.gov/cdrh/postmarket/mdpi-report-1106.html.
2. Susan Gardner, "Medical Device Safety" (presentation to the Massachusetts Medical Device Industry Council, Boston, December 1, 2006).
3. Susan Gardner and Marilyn Flack, Designing a Medical Device Surveillance Network [report to Congress] (Rockville, MD: Center for Devices and Radiological Health, FDA, 1999); available from Internet: www.fda.gov/cdrh/postsurv/medsun.html#4.1.
4. Quality System Regulation, Code of Federal Regulations, 21 CFR 820.
5. Medical Device Reporting Regulation, Code of Federal Regulations, 21 CFR 803.
6. "FDA Electronic Submission Gateway (ESG) User Guide" (Rockville, MD: FDA, 2006); available from Internet: www.fda.gov/esg/user guide/WebHelp/helpfile.htm.
7. "MedWatch Online Voluntary Reporting Form 3500," (Rockville, MD: FDA, [cited 18 July 2007]); available from Internet: www.accessdata.fda.gov/scripts/medwatch.
8. "CeSub eSubmitter" [downloadable electronic submission software], (Rockville, MD: Center for Devices and Radiological Health, FDA, 2007 [cited 18 July 2007]); available from Internet: www.fda.gov/cdrh/cesub.
Michael Gram is a business development manager focused on the medical device industry for Maxiom Group (Waltham, MA). Copyright ©2007 MX
About the Author(s)
You May Also Like