MPMN 25th Anniversary Coverage
December 14, 2010
 In the 19th century, Florence Nightingale was a pioneer in showing a correlation between cleanliness of treatment facilities and reduction of infection rates. Since then, however, infection-prevention methods in the clinical setting have evolved beyond just keeping facilities clean. As pressure is applied by both consumers and governmental bodies to solve the challenge of hospital-acquired infections (HAIs), medical device manufacturers are turning to new technologies, such as embedded silver antimicrobials, that can be incorporated into an overall infection control strategy.
In the 19th century, Florence Nightingale was a pioneer in showing a correlation between cleanliness of treatment facilities and reduction of infection rates. Since then, however, infection-prevention methods in the clinical setting have evolved beyond just keeping facilities clean. As pressure is applied by both consumers and governmental bodies to solve the challenge of hospital-acquired infections (HAIs), medical device manufacturers are turning to new technologies, such as embedded silver antimicrobials, that can be incorporated into an overall infection control strategy.
Although there have been some early innovators employing embedded silver-based antimicrobials, use of this novel technology is still in its nascent stages in the medical device industry. Adoption of embedded antimicrobials in medical facilities, for example, was first introduced primarily in environmental high-touch surfaces, such as door hardware, furniture, uniforms, and flooring. But increased public scrutiny of the high infection rates at healthcare facilities, coupled with the rise of antibiotic-resistant strains, thrust medical devices under the microscope as another source of bacterial growth and related infections.
Embedded silver antimicrobials emerged as a potential solution. The beauty of silver as an antimicrobial lies in its inherent broad-spectrum efficacy in combating both Gram-positive and Gram-negative bacteria with no known resistance problems throughout its long history of use. It does so by sterilizing, suffocating, and starving the bacterial cell.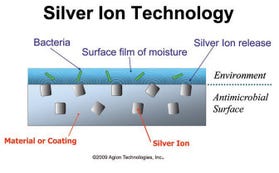
Companies began to capitalize on these benefits in the 1990s by commercializing silver antimicrobials for plastics and coatings. And while companies such as Vygon were among the first in 2000 to implement silver-based antimicrobials on catheters, it was the 2008 Centers for Medicaid and Medicare Services (CMS) changes to reimbursement policy that created an economic driver for antimicrobial implementation and use. This particular event continues to drive the search for new methods of controlling bacteria and biofilm growth on the medical products cited by CMS as devices of concern, including CVC catheters, vascular products, and respiratory devices.
These reimbursement changes--in conjunction with the convergence of such factors as the availability of FDA-approved silver antimicrobial-treated devices, the continuation of studies demonstrating the link to reduced infection rates, the economic and socioeconomic drivers, and the OEM awareness of the availability of such technologies--are creating growth in this market on all fronts. And as healthcare environments zero in on the root causes of infection, there will continue to be devices that are tied to higher incidence of bacteria or biofilm formation. Clearly, these devices of concern already include catheters and respiratory devices, and the penetration of antimicrobial use on these devices alone is still relatively small. Device manufacturers of such products as implants will most likely be adopters of this type of technology moving forward.
But while multiple medical device OEMs have published studies with data indicating infection-rate reductions on such products, medical devices embedded with antimicrobials are used as part of an overall infection-control strategy. So, as one piece of a multifaceted solution, it can be tough for facilities to cite antimicrobial-embedded devices as a specific cause of reduction in infection rates.
Hand washing, cleaning procedures, stringent infection control practices, and antibiotic therapies will always play a crucial role in HAI prevention. However, use of such embedded technologies as silver antimicrobials is just one more facet of advancement in combating this growing global problem.
Paul Ford is CEO of Agion (Wakefield, MA). Visit the company's Web site at www.agion-tech.com for more information.
You May Also Like
.png?width=300&auto=webp&quality=80&disable=upscale)

