December 1, 2006
2006 MEDTECH SNAPSHOT
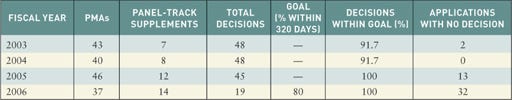
Because FDA has changed its goals with regard to complying with MDUFMA, some information on this page does not correlate with previous years. For premarket approvals (PMAs), cycle goals apply to 75% of submissions received in FY 2005, 80% of those received in 2006, and 90% of submissions in 2007. FDA's goals state that it will make decisions on 80% of PMAs received in 2006.
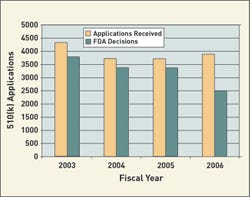
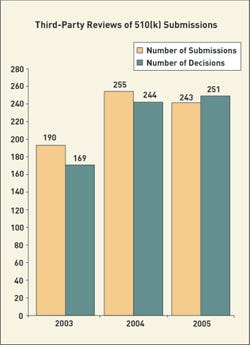
Likewise, premarket notifications (510(k)s) have a new organization that makes it difficult to compare them with previous years. FDA has said it will have a decision for 75% of submissions received in FY 2005 and FY 2006. For FY 2005, FDA plans to apply these goals to 70% of the submissions. In 2006, it will include 80% of those submissions, and in 2007 the goals will apply to 90%.
Click images and tables to enlarge:
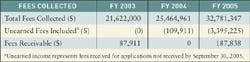
|
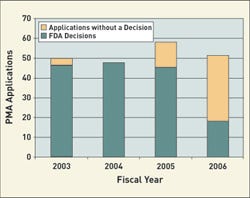
FDA actions on PMA applications and panel-track supplements. Figures for 2006 are through September 2006. FDA expects to meet the goal for 2006. Source: FDA Quarterly Update on Progress Towards Meeting MDUFMA Performance Goals. |
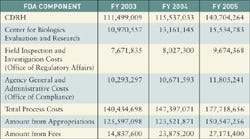
|
|
Copyright ©2006 Medical Device & Diagnostic Industry
You May Also Like