Originally Published MDDI December 2005 2005 MEDTECH SNAPSHOT
December 1, 2005
Originally Published MDDI December 2005
2005 MEDTECH SNAPSHOT
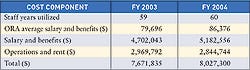
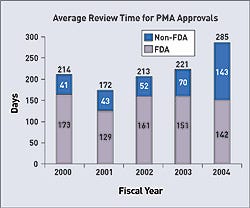
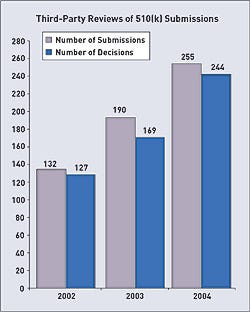
The dramatic increases in the user fees mandated by the Medical Device User Fee and Modernization Act of 2002 (MDUFMA) have sparked much discussion and controversy during the last few years. In response, FDA prepared a report that examines the unit costs of the agency's medical device review process. The unit costs are, in effect, the average costs for FDA to review various types of applications during a particular period of time.
Other user-fee programs, such as the Prescription and Animal Drug User Fee Programs, differ from MDUFMA significantly, according to the report. Those user-fee pricing mechanisms have resulted in lower fees per application because the majority of fee revenue is collected from other mechanisms, including manufacturing establishments and product and sponsor fees. MDUFMA user fees, however, are derived solely from applications.
|
| ||||
|
| ||||
|
|
Copyright ©2005 Medical Device & Diagnostic Industry
You May Also Like