September 1, 1998

Refractive Surgical Device Benefits from Plastic Construction
Because of their wide range of properties, ability to be sterilized, and economical benefits, plastics are being used in place of metals in a number of medical devices. For Refractive Technologies Inc. (Cleveland), selecting the appropriate material was key to the viability of developing the first predominantly plastic microkeratome for refractive ophthalmological procedures. The new device, which provides ease of use, accuracy, and safety while allowing for significant parts consolidation and cost containment, is made almost entirely of a polycarbonate resin.Developed by Alex Dybbs of Refractive Technologies, the FLAPmaker is an innovative, single-use, disposable microkeratome device designed to make a flaplike cut (about 0.16 mm) with a metal blade into the top layer of the cornea for refractive eye surgeries that involve the use of a laser. In these procedures, which are commonly performed to correct nearsightedness, the surgeon folds the flap back, applies laser sculpting techniques to reshape the cornea, and then puts the flap back in place. Dybbs himself was the first patient to receive this surgery using the FLAPmaker.
A Need for Improvement
As the popularity of refractive procedures has grown, there has been a need to improve existing metal microkeratomes, which require resterilization, extensive assembly, and precise blade positioning before every procedure. "The metal instrument dictates a high level of maintenance and knowledgeable technical support to ensure proper use," explains Dybbs. "There is also a learning curve involved with understanding how to correctly assemble the device and the safety risks involved with not assembling it correctly. The FLAPmaker was designed to eliminate these problems."
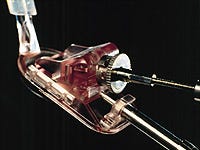 The FLAPmaker is the first predominantly plastic microkeratome for refractive ophthalmological procedures. Its manufacturer, Refractive Technologies, found that using plastic has helped make the device safer and easier to use than metal microkeratomes.
The FLAPmaker is the first predominantly plastic microkeratome for refractive ophthalmological procedures. Its manufacturer, Refractive Technologies, found that using plastic has helped make the device safer and easier to use than metal microkeratomes.
The FLAPmaker is molded out of a clear polycarbonate to allow the surgeons to easily see the cornea during the procedure. The device is gearless for smoother operation and features a fixed depth plate to reduce the risk of unwanted cuts to the cornea. It also saves time since it is preassembled, unlike metal microkeratomes, which contain many tiny parts that must be assembled before each procedure.
"The FLAPmaker device is identical in principle to other microkeratomes, but through the use of plastic, it's safer, more ergonomic, easier to take care of, and easier to use," explains Jeffrey B. Robin, a refractive surgeon and medical director of the NuVista Refractive Surgery and Laser Center in Cleveland. Robin is also president of the International Society of Refractive Surgery and has served as an adviser to Refractive Technologies throughout the development of the FLAPmaker.
According to Dybbs, surgeons performing these types of procedures are most concerned with the consistency of the instrument. For example, with existing microkeratomes, care must be taken to set the depth plate precisely to avoid making a larger cut in the cornea than is necessary. This complication is not possible with the FLAPmaker, as it features a verification of the depth plate "gap" and a microscopic photograph of the blade. "Each unit is inspected through quality control procedures to assure surgeons of the device's accuracy. The claim specification included with each unit lists the margin of error with regard to the thickness of the cut and the quality of the blade," explains Dybbs.
Surgeons also want to ensure that the flap cut does not go completely across the cornea, creating a free cap, and requiring additional recovery time. "Metal designs have a stop screw to prevent the blade from completely disconnecting the flap from the eye, but again, if the instrument is not set up correctly, this is a useless function," notes Robin. "The FLAPmaker is preset to go only a certain distance, thus eliminating these situations."
Choosing a Plastic
"Without a doubt, plastic was the material of choice for this application, due to its strength, its design flexibility, and its ability to allow the device to be delivered to the surgeon preassembled and to be disposed of after use," says Dybbs.
Refractive Technologies contacted Dow Plastics (Midland, MI) and requested a plastic with excellent optical properties and resistance to gamma sterilization. The material also had to be able to run in existing mold designs. Dow worked with Refractive Technologies to provide the selection, technical support, and material training assistance required to meet these needs. After conducting several tests, Refractive Technologies selected Calibre MegaRad polycarbonate resins.
"Calibre resins meet the processing requirements of the device while allowing the molder to maintain consistent dimensions in the finished part to within ±0.01% of the specified tolerances," says Karen Winkler, a senior applications development engineer at Dow Plastics.
The Benefits
According to Winkler, Calibre MegaRad resins were developed in direct response to the need for improved clarity in medical devices that undergo high-energy radiation (gamma or electron beam). Calibre MegaRad resins offer clarity, dimensional stability, high impact strength, and heat resistance. Available in a range of melt-flow rates, these resins enable the molder to design specific properties into the finished parts.
According to Dybbs, while the properties and processing characteristics of the polycarbonate resins used in the FLAPmaker were primary concerns, minimizing the cost and complexity of the device was also important. "The finished FLAPmaker microkeratome consists of only five plastic parts compared to the approximately 25 in existing metal microkeratomes," says Dybbs. "The use of plastic has made the FLAPmaker an economical alternative to existing metal microkeratomes, which can cost up to six times more."
Additionally, the flaps made with the FLAPmaker are thicker, more consistent, and of a better quality. "The flaps are smoother and fit back over the eye much easier than a thinner, uneven flap. This also helps speed up recovery time," says Robin.
To date, the FLAPmaker has been used successfully by more than 28 surgeons around the world. The device, which went from concept to prototype in four months and was developed in just under a year, is expected to receive FDA approval this year.
MPMN is actively seeking success stories like this. If your company has one to tell, please contact associate editor Karim Marouf at 3340 Ocean Park Blvd., Ste. 1000, Santa Monica, CA 90405; 310/392-5509 or E-mail [email protected]
You May Also Like


