March 2, 2011
|
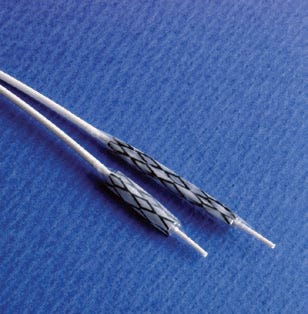
A specialized heat-curable binder from NuSil enables CI Medical's radiopaque ink to adhere to silicone-based medical devices. |
Bonding radiopaque inks to silicone substrates has historically been challenging because of the elastomer's low surface energy. And while manufacturers have circumvented this obstacle by extruding or molding radiopaque material directly into the silicone, the addition of this nonfunctional filler can diminish the material's physical properties. To meet this challenge head on, CI Medical Inc. has enlisted the support of NuSil Technology to develop a specialized heat-curable binder that enables the company's radiopaque ink to adhere to silicone-based medical devices.
"In order to print on silicone, you must use a silicone-based ink that can bond sufficiently to the substrate and move with it--i.e., be flexible," explains Bruce Mahan, engineering manager at CI Medical. "When an opacifier--in this case, tungsten--is added to the base ink, traditional binders do not have enough surface to complete the bond, causing the radiopaque ink to delaminate when flexed." The company's goal, according to Mahan, was twofold: to develop an ink that would print well and bond permanently when loaded with a sufficient dose of tungsten to make it appear under fluoroscopy.
NuSil responded by developing a silicone-based binder material to accommodate CI Medical's radiopaque filler, remarks Brian Reilly, NuSil's product director for healthcare materials. "The challenge was to develop a material that meets specific viscosity and volatility requirements. It also had to satisfy specific work-time conditions, meaning that once the filler and binder were mixed, the end material had to demonstrate appropriate rheology characteristics for a given period of time, including flowability, pourability, and coatability." The material couldn't be pastelike, Reilly adds, because that would not lend itself to being used for marking very narrow sections of a silicone component.
In addition to exhibiting a range of physical characteristics, the radiopaque ink had to be biocompatible for use in medical implants. "Besides all the physical-property testing, we did a per-lot tissue culture on the elastomer," Reilly says. "We formulated it with the notion in mind that it would have to be supported from a biological standpoint." Thus, the company relied on ingredients that it had employed in other, similar silicone elastomer systems for short- and long-term implant applications.
The radiopaque ink can be printed on virtually any silicone-based medical device, including catheters, according to Mahan. It can also be printed on molded parts and sheets of material that are wrapped around implants or on pad-printable silicone material. The ink itself, including the binder, comes with a Master File. The only variable ingredient is tungsten, which contrasts with the silicone device and the tissue or bone around it. "By baking the ink formulation at 150°C, we get a great bond," Mahan concludes.
CI Medical Inc.
Norton, MA
www.cimedical.com
NuSil Technology
Carpinteria, CA
www.nusil.com
You May Also Like