May 1, 2001
Originally Published MPMN May 2001
Presenting the 2001 Medical Design Excellence Awards
The Medical Design Excellence Awards (MDEA) is the only awards program devoted exclusively to recognizing contributions and advancements in the design of medical products. An impartial, multidisciplinary 10-member jury with expertise in biomedical engineering, human factors, industrial design, medicine, nursing, diagnostics, and medical packaging evaluated more than 80 products submitted to the MDEA 2001 competition. The 28 finalists representing the 10 categories are profiled here.
The MDEA program is organized by Canon Communications llc and sponsored by MPMN's sister publication, Medical Device & Diagnostic Industry magazine. Corporate sponsorship for the 2001 MDEA competition is being provided by MedSource Technologies (Minneapolis), a firm that specializes in full-service outsourcing and complete project management for medical device manufacturers; DuPont Tyvek (Wilmington, DE), a supplier of sterile packaging materials for medical products; Agion Technologies LLC (El Segundo, CA), a provider of antimicrobial surface treating services; Battelle (Columbus, OH), a company that offers R&D and project development services; Avail (Dallas), a contract manufacturer of medical disposable products; Colorado MEDtech (Boulder, CO), a full-service contract manufacturer and product developer of medical devices and equipment; and IBA Medical Sterilization & Analytical Labs (Chicago), a supplier of sterilization and laboratory services.
The winning products will be displayed at the Medical Design and Manufacturing East Exposition, June 5–7, 2001, in New York City. For more information, call Canon Communications at 310/445-4200 or visit the program's Web site at http://www.mdeawards.com.
Critical Care and Emergency Products
Life Support for Trauma and Transport
Individualized, portable system provides flexible, continuous care
The Life Support for Trauma and Transport (LSTAT) system is a networked intensive-care unit and surgical table in a smart patient platform that measures only 5 in. thick. It comprises a state-of-the-art defibrillator, ventilator, suction device, three-channel fluid and drug-
infusion pump, point-of-care body chemistry analyzer, and patient monitoring subsystem. These medical subsystems are integrated onto a common information architecture that captures, stores, and transmits continuous, real-time, multidevice patient data. Integrated Medical Systems Inc., Signal Hill, CA.
Radical Signal-Extraction Technology pulse oximeter
Pulse oximeter noninvasively monitors oxygen saturation and pulse rate
The signal-extraction technology pulse oximeter was designed for continuous noninvasive monitoring of oxygen saturation of arterial hemoglobin and pulse rate for adult, pediatric, and neonatal patients in hospital and home environments. It consists of circuit boards with analog components and DSP chips with patented algorithms, an LCD, a patient cable and sensor connection, a medical-grade power supply, a serial communications port, and analog output and nurse-call interfaces. It can be used as a stand-alone pulse oximeter, a handheld oximeter for spot checking and transport monitoring, and to upgrade pulse oximetry in existing patient monitors. Masimo Corp., Irvine, CA; I.N. Inc., Los Alamitos, CA.
XCalibur Mobile Transporter
Composite ambulance cot enhances prehospital emergency care
 With a 600-lb capacity, the XCalibur accommodates very heavy patients while providing ergonomic benefits to its operators. Control mechanisms are concealed inside hollow composite tubing to increase safety and reduce required maintenance. The XCalibur is made of multilayer resin and glass composites held together with epoxy adhesives. Using composite materials, the designers were able to make the cot aesthetically simple and cleanable with subassemblies that can be easily replaced using common tools. Ferno-Washington Inc., Wilmington, OH.
With a 600-lb capacity, the XCalibur accommodates very heavy patients while providing ergonomic benefits to its operators. Control mechanisms are concealed inside hollow composite tubing to increase safety and reduce required maintenance. The XCalibur is made of multilayer resin and glass composites held together with epoxy adhesives. Using composite materials, the designers were able to make the cot aesthetically simple and cleanable with subassemblies that can be easily replaced using common tools. Ferno-Washington Inc., Wilmington, OH.
Dental Instruments, Equipment, and Supplies
BriteSmile 2000 tooth-whitening system
System requires less time than conventional methods
The BriteSmile 2000 incorporates fiber-optic light emitters in conjunction with a gel for a noticeable whitening effect after three applications. Fiber-optic cables are carried inside an articulating arm to bring the light from the cabinet to customized light emitters that shine in a constrained pattern. Multiple emitters illuminate only the teeth that require whitening to reduce treatment time to 11Ž2 hours. Ideo Chicago, Evanston, IL; BriteSmile Inc., Walnut Creek, CA.
WaterPik Flosser
Lightweight handheld device removes dental plaque
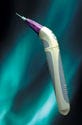 A single-use nylon filament enables the WaterPik to gently reach between teeth and below the gum line to remove plaque associated with gingivitis. The unit consists of a soft-grip handle that operates on one AA battery, a cartridge containing a month's supply of replaceable flossing tips, and a stand or charging base. Also available is a set of color-coded snap-on hygienic sleeves to prevent family members from accidentally sharing devices. The product is designed to be easier to use than dental floss because it comfortably reaches into areas where plaque forms and the tip does not break off between teeth. Volan Design, Boulder, CO; WaterPik Technologies, Ft. Collins, CO.
A single-use nylon filament enables the WaterPik to gently reach between teeth and below the gum line to remove plaque associated with gingivitis. The unit consists of a soft-grip handle that operates on one AA battery, a cartridge containing a month's supply of replaceable flossing tips, and a stand or charging base. Also available is a set of color-coded snap-on hygienic sleeves to prevent family members from accidentally sharing devices. The product is designed to be easier to use than dental floss because it comfortably reaches into areas where plaque forms and the tip does not break off between teeth. Volan Design, Boulder, CO; WaterPik Technologies, Ft. Collins, CO.
Finished Packaging
Kabiven multichamber parenteral nutrition packaging system
Package is designed to hold a total nutrient admixture in a single container
The Kabiven parenteral nutrition package holds a mixture of fat, amino acids, glucose, and electrolytes in three chambers separated by inner seals. This design simplifies preparation and allows room-temperature storage for 24 months prior to mixing. Peelable seals make it possible to mix the product without exposing the solutions to the environment. The overall packaging concept seeks to minimize environmental impact, toxic emissions or by-products, and waste volume. Fresenius Kabi, Uppsala, Sweden.
Prompt L-Pop dental adhesive system
Etching, priming, and bonding can be performed in a single step
 The packaging of the Prompt L-Pop divides contents into three separate compartments. It is suitable for bonding between dentin and enamel to composite filling materials and fissure sealants, as well as for orthodontic bracket attachment. The single-patient packaging eliminates the need for bottles and evaporation while reducing cross-contamination. ESPE Dental AG, Seefeld, Germany.
The packaging of the Prompt L-Pop divides contents into three separate compartments. It is suitable for bonding between dentin and enamel to composite filling materials and fissure sealants, as well as for orthodontic bracket attachment. The single-patient packaging eliminates the need for bottles and evaporation while reducing cross-contamination. ESPE Dental AG, Seefeld, Germany.
General Hospital Devices and Therapeutic Products
Acapella chest physical therapy device
Device combines benefits of positive-pressure therapy and airway vibrations
 Designed to mobilize pulmonary secretions, the Acapella chest physical therapy (CPT) device is intended for patients with lung disease and associated secretory problems. It is easier to tolerate and is less time-consuming than other CPT systems and facilitates the opening of airways. The vibratory positive-pressure (PEP) therapy system functions independently of gravity controls and meets the needs of patients with low-pressure and low-flow constraints. The device can be used with a standard mouthpiece, a mask, or a TheraPEP pressure port and gauge for precise measurements. It contains a one-way inspiratory valve that allows inhalation and exhalation without removing the product from the patient's mouth. Product Genesis Inc., Cambridge, MA; DHD Healthcare, Wampsville, NY.
Designed to mobilize pulmonary secretions, the Acapella chest physical therapy (CPT) device is intended for patients with lung disease and associated secretory problems. It is easier to tolerate and is less time-consuming than other CPT systems and facilitates the opening of airways. The vibratory positive-pressure (PEP) therapy system functions independently of gravity controls and meets the needs of patients with low-pressure and low-flow constraints. The device can be used with a standard mouthpiece, a mask, or a TheraPEP pressure port and gauge for precise measurements. It contains a one-way inspiratory valve that allows inhalation and exhalation without removing the product from the patient's mouth. Product Genesis Inc., Cambridge, MA; DHD Healthcare, Wampsville, NY.
Goldenberg Snarecoil needle
Disposable needle reduces patient anxiety and pain
The Goldenberg Snarecoil needle is a sterile, single-use device that is designed for obtaining bone-marrow specimens in an outpatient setting under local anesthesia. It consists of two sections: a handle constructed of injection-molded polycarbonate plastic components, and a needle made of stainless-steel tubing and a sharpened wire. An inner snare mechanism allows the physician to capture the specimen after insertion with a simple movement of the device's lever. The needle is then removed with a minimal amount of force and without causing specimen distortion. Ranfac Corp., Avon, MA; Alec S. Goldenberg, MD, Bellevue Hospital Center, New York City.
Grab n Go III portable medical oxygen system
Device combines an oxygen cylinder, regulator, and content gauge
The Grab n Go III was designed to simplify the administration of portable medical oxygen while improving safety through the elimination of exposure
to high-pressure gas connections. The precision-machined unit has fewer parts than a conventional portable oxygen system. Components are integrated to provide value through ease of use and safety, while reducing overall cost to end-users. Praxair Inc., Danbury, CT; Western Medica, Westlake, OH; Lewellen Design Inc., Wooster, OH.
Protectiv Acuvance IV safety catheter
Safety feature provides needlestick injury protection
Designed for the administration of medically prescribed fluids, the Protectiv Acuvance IV safety catheter is a sterile, nonpyrogenic, nontoxic, single-use IV device. The catheter features a unique needle-holder design to facilitate handling of the device during insertion and threading into the vein. Almost the same size as conventional IVs, the Protectiv Acuvance does not require changes in insertion technique and can be redirected to enter the vein, if needed. Ethicon Endo-Surgery Vascular Access, Cincinnati, OH; BioPlexus, Tolland, CT.
Implant and Tissue-Replacement Products
Ancure endograft system
Abdominal aortic aneurysm treatment is less traumatic
 The Ancure surgical procedure uses a catheter to insert a sheath through the femoral artery in the leg, a less-invasive procedure that causes fewer traumas than traditional surgical techniques. The device's graft implant passes through a 5-mm aperture and is then positioned, deployed, and fastened remotely. The delivery technique involves removing the implant jacket, exposing the ipsilateral attachment system, using pull rings to release the implant, controlling its movement, and sealing it. Numbered safety tabs prevent out-of-sequence deployment. Stirling Design, Aptos, CA; Guidant Corp., Indianapolis.
The Ancure surgical procedure uses a catheter to insert a sheath through the femoral artery in the leg, a less-invasive procedure that causes fewer traumas than traditional surgical techniques. The device's graft implant passes through a 5-mm aperture and is then positioned, deployed, and fastened remotely. The delivery technique involves removing the implant jacket, exposing the ipsilateral attachment system, using pull rings to release the implant, controlling its movement, and sealing it. Numbered safety tabs prevent out-of-sequence deployment. Stirling Design, Aptos, CA; Guidant Corp., Indianapolis.
Helex septal occluder
Prosthesis minimizes invasive closure of atrial septal defects
A permanently implanted prosthesis is composed of an implantable occluder that is premounted on a 9-F delivery system and a preshaped delivery catheter for access to atrial septal defects that eliminates the need for a long intruder sheath. Within the 9-F delivery catheter is a control catheter, which pushes the device during delivery, and an integral retrieval cord that allows device withdrawal during loading and retrieval. Both catheters are supplied with radiopaque markers at their distal tips for fluoroscopic visualization during device deployment. A cardiologist can reposition or remove a malfunctioning device without compromising patient safety. W. L. Gore and Associates, Flagstaff, AZ.
Nucleus 24 Contour cochlear implant
Implant benefits those with severe to profound hearing loss
The Nucleus 24 Contour is an implant with a permidiolar electrode array that safely places stimulating electrodes adjacent to the modiolar wall of the cochlear without the use of invasive bands or positioners. The implant operates by receiving sound through a speech processor, then transforming it into electrical impulses that are transferred through the electrode array to stimulate the ganglion nerve cells in the modiolus. To achieve optimal access to the auditory nerve, a conical cavity is drilled in the temporal bone with a tiny entrance that can accommodate insertion of the implant. Cochlear Ltd., Sydney, Australia.
In Vitro Diagnostics
Benchmark automated histology staining system
Automated system stains tissue samples on glass microscope slides
 The Benchmark automates the labor-intensive steps required in baking, dewaxing, cell conditioning, and staining tissue for cancer and infectious disease diagnosis. The slides are processed with various reagents that remove embedded paraffin, pretreat tissue to expose targets, and bind and detect specific antigens or DNA and RNA sequences with localized color change or fluorescence. The system can control slide temperature and evaporation, minimize solvent exposure, control multiple instruments through a single user interface, and read patient sample and reagent bar codes. Ventana Medical Systems Inc., Tucson, AZ.
The Benchmark automates the labor-intensive steps required in baking, dewaxing, cell conditioning, and staining tissue for cancer and infectious disease diagnosis. The slides are processed with various reagents that remove embedded paraffin, pretreat tissue to expose targets, and bind and detect specific antigens or DNA and RNA sequences with localized color change or fluorescence. The system can control slide temperature and evaporation, minimize solvent exposure, control multiple instruments through a single user interface, and read patient sample and reagent bar codes. Ventana Medical Systems Inc., Tucson, AZ.
EScreen drugs-of-abuse testing system
A networked point-of-care system designed for drug testing
The eScreen includes eCup, a specimen cup with a smart lid, and an optical Internet appliance that scans, reads, and writes drugs-of-abuse test assay results and sends the results confidentially via a secure Internet connection. The fluid sample is transferred from the cup to the test chambers by means of a plunger that acts directly on a portion of the urine sample to force it between a double-walled stem to the lid and then distribute it into three test chambers. eScreen Inc., Los Angeles.
Over-the-Counter/Self-Care Products
TravelMate female urinary device
A noninvasive female urinary device reduces need to self-catheterize prior to travel
 TravelMate is an alternative to female urinals and traditional urinary catheters that is noninvasive and reduces the risk of urinary tract infection. It consists of a piece of polyethylene that conforms to shape variations in the area surrounding the urethral orifice. It creates a comfortable leak-free seal between the device and the body but is small enough to be easily positioned even while sitting in a wheelchair or standing. Caring Hands Inc., Hayden, ID; JB Engineering Inc., Pomona, CA.
TravelMate is an alternative to female urinals and traditional urinary catheters that is noninvasive and reduces the risk of urinary tract infection. It consists of a piece of polyethylene that conforms to shape variations in the area surrounding the urethral orifice. It creates a comfortable leak-free seal between the device and the body but is small enough to be easily positioned even while sitting in a wheelchair or standing. Caring Hands Inc., Hayden, ID; JB Engineering Inc., Pomona, CA.
Radiological and Electromechanical Devices
Beta-Cath interventional cardiology device
Prevents or treats restenosis following balloon angioplasty
 To prevent vessel closure, the Beta-Cath delivers a prescribed dose of beta radiation to the coronary artery vessel wall from inside the coronary artery via a catheter-delivery system. The transfer device stores, shields, and delivers beta radiation through individual sealed sources that travel to the artery, then return to the device via a fluid-hydraulic delivery method. A small multilumen single-use delivery catheter allows radiation to reach the treatment site while preventing it from touching body tissues. Novoste Corp., Norcross, GA; Innovation Factory, Norcross, GA.
To prevent vessel closure, the Beta-Cath delivers a prescribed dose of beta radiation to the coronary artery vessel wall from inside the coronary artery via a catheter-delivery system. The transfer device stores, shields, and delivers beta radiation through individual sealed sources that travel to the artery, then return to the device via a fluid-hydraulic delivery method. A small multilumen single-use delivery catheter allows radiation to reach the treatment site while preventing it from touching body tissues. Novoste Corp., Norcross, GA; Innovation Factory, Norcross, GA.
DirectView CR800 system
System facilitates wide electronic x-ray distribution
A radiographic digital image capture and processing system utilizes exam-room exposure sources and cassette-based techniques to create electronic records of patients' images for future reference. Typical imaging applications include chest x-rays, bone x-rays, and soft tissue images. The DirectView CR800 has a remote operations panel that allows multiple units to be placed throughout the imaging environment to improve workflow and patient care. Eastman Kodak Co., Rochester, NY.
Echo-Coat ultrasound needles
Needle shafts are visible under ultrasound imaging
Ultrasound needles are coated to render the shaft visible with ultrasound imaging, enabling the devices to be used under ultrasound guidance during aspirations, biopsies, localizations, and amniocentesis procedures. Echo-Coat ultrasound coating is a layered polyurethane system when applied to 304-stainless-steel needles, the porous microstructure entraps tiny bubbles of air to enhance the echogenicity regardless of the needle angle relative to the ultrasound beam. STS Biopolymers Inc., Henrietta, NY.
Rehabilitation and Assistive-Technology Products
Claro and WatchPilot digital hearing instruments
Digital hearing aid system is remote controlled
Claro uses two microphones to create digital response and digital perception processing to emulate normal human hearing. An AudioZoom function
creates a directional response, allowing the user to increase sound levels of speech while decreasing background noise. The digital perception processing uses 20 overlapping and interdependent channels to process sound like the normal ear. A fine-scale noise canceller scans channel signals and identifies and increases speech signals and reduces other noises. Phonak, Warrenville, IL.
Helios personal oxygen system
High-efficiency liquid oxygen system designed for ambulatory use
 A portable oxygen system consists of a 46-L reservoir and a pneumatic 4:1 conserver device, which allow for a good combination of duration and small size. The unit weighs 3.4 lb when full. The double-walled oxygen bottles have multilayered, glass paper and aluminum foil insulation contained in a vacuum to reduce heat transfer and cryogenic evaporative losses. The volume of oxygen supplied to the user is delivered in a pulse at the beginning of patient inspiration to maximize efficacy. Tyco Healthcare Puritan-Bennett, Indianapolis; Omnica Corp., Irvine, CA.
A portable oxygen system consists of a 46-L reservoir and a pneumatic 4:1 conserver device, which allow for a good combination of duration and small size. The unit weighs 3.4 lb when full. The double-walled oxygen bottles have multilayered, glass paper and aluminum foil insulation contained in a vacuum to reduce heat transfer and cryogenic evaporative losses. The volume of oxygen supplied to the user is delivered in a pulse at the beginning of patient inspiration to maximize efficacy. Tyco Healthcare Puritan-Bennett, Indianapolis; Omnica Corp., Irvine, CA.
Pathfinder prosthetic foot
Provides a wide range of motion and optimizes the wearer's gait
Intended for lower-extremity amputees who have moderate to high activity levels, the Pathfinder prosthetic foot combines shock absorption and energy storage capabilities. To provide an optimal gait, the device incorporates a triangular design, a closed shape, and three main sides that act together synergistically. The sides—a pneumatic heel spring, composite toe springs, and a composite foot plate—are connected at three main joints: toe connectors, the heel connector, and the proximal connector. Ohio Willow Wood Co., Mt. Sterling, OH.
Surgical Equipment, Instruments, and Supplies
Aortic connector system
Implant allows minimally invasive bypass surgery method
The Aortic connector system creates the proximal anastomosis of an aortic autologus vein graft and eliminates the need for hand suturing. The system comprises a nitinol implant, injection-molded ABS delivery device, and stainless-steel release tubes. The aortic delivery device has a pipette configuration to allow greater accuracy and reduced hand movement. Redgroup, Minneapolis; St. Jude Medical, St. Paul, MN.
InsideView video projection method
Surgeons can view the internal operative site and their hands simultaneously
An advanced video projection method displays high-resolution images on a sterile disposable screen positioned within the surgical field. The system's 3.15 million-pixel Sony monitor is equipped with a zoom lens that is held securely within an optical-fixture mechanism. The disposable EtO-sterilized screen kits are made of high-grade, lightweight optical plastics. Applications include endoscopy, radiological procedures, and urologic, gynecologic, and cardiac interventions. LSI Solutions, Rochester, NY.
Mammotome handheld breast biopsy system
Multiple biopsy samples are collected with a single needle insertion
The Mammotone system is a minimally invasive, highly accurate mobile instrument for helping doctors diagnose breast cancer at the earliest stages. The probe is inserted once into the patient's breast via a small incision. Once inserted and positioned via ultrasonic imaging, it uses vacuum aspiration and an internal rotating cutter to collect multiple samples up to eight times the weight of those obtained using traditional spring-loaded biopsy equipment. A metal tissue marker can be deployed through the probe into the sampled biopsy making it easier to locate the lesion site for postprocedure mammographic diagnosis. Plexus Corp., Neenah, WI; Herbst Lazar Bell, Chicago; Dow Plastics, Midland, MI; Ethicon Endo-Surgery Inc., Cincinnati.
Neptune waste-management system
Evacuates, collects, and disposes of hazardous fluids and gases
 The Neptune waste-management system is designed to dispose of hazardous surgical fluid waste and smoke from electrocautery or laser devices. The system consists of a rover that can collect up to 20 L of fluid at one time and a docking station that sterilizes collected fluid prior to disposal. The system also includes a traditional suction canister-tree combined with a smoke evacuator, an optional power IV pole to aid in the delivery of irrigation fluid waste, and a digital display that monitors fluid absorption. American Immuno Tech Inc., Costa Mesa, CA; Bio-Medical Devices Inc., Costa Mesa, CA; Stryker Instruments Div. of Stryker Corp., Kalamazoo, MI.
The Neptune waste-management system is designed to dispose of hazardous surgical fluid waste and smoke from electrocautery or laser devices. The system consists of a rover that can collect up to 20 L of fluid at one time and a docking station that sterilizes collected fluid prior to disposal. The system also includes a traditional suction canister-tree combined with a smoke evacuator, an optional power IV pole to aid in the delivery of irrigation fluid waste, and a digital display that monitors fluid absorption. American Immuno Tech Inc., Costa Mesa, CA; Bio-Medical Devices Inc., Costa Mesa, CA; Stryker Instruments Div. of Stryker Corp., Kalamazoo, MI.
Tissuebond applicator and Tissuemed 180 light source system
A light activates applied tissue sealant
The Tissuebond applicator contains a solution composed of porcine albumin glycerol, water for injection, and methylene blue that allows the surgeon to see where the sealant has been applied and stimulates the bonding process when the light is applied to it. The applicator is a disposable, finger-controlled penlike delivery system that dispenses an accurate bead of adhesive onto the desired tissue area. The light source is controlled by a foot switch and delivers a cold-filtered light beam through a handheld wand. DCA Design Consultants, Warwick, UK; Tissuemed Ltd., Leeds, UK.
Copyright ©2001 Medical Product Manufacturing News
You May Also Like


