New polymers feed a market hungry for enhanced performance characteristics, aesthetics, and sustainable resources
September 14, 2009
Originally Published MPMN September 2009
PRODUCT UPDATE
Plastics Providers Respond to Demand for Supply Continuity and Sustainability
New polymers feed a market hungry for enhanced performance characteristics, aesthetics, and sustainable resources
Stephanie Steward
|
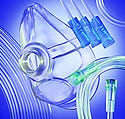
PolyOne has launched biopolymer compounds for use in such applications as tubing, IV pumps, respiratory equipment, and medical device electronics housings. |
Many plastics suppliers timed the release of new materials this year with NPE2009, the exposition produced by SPI, the Plastics Industry Trade Association (Washington, DC; www.plasticsindustry.org). And the buzzword of this year’s show was bioplastics, which reflects the increase in interest in materials that are derived from renewable resources. Additionally, new polymer materials reflect efforts of suppliers to meet customer demand for enhanced performance characteristics for applications ranging from valves to medical device packaging. Without sacrificing performance, OEMs are also seeking to enhance their products with coloring options, a trend that has as much to do with appealing to the consumers as it does with branding. The question for OEMs now is not what materials are available, but, as Larry Johnson, global marketing director, healthcare, for PolyOne (Cleveland; www.polyone.com) put it: What’s your objective?
The Biopolymer Bandwagon
The increased industry interest in bioplastics was reflected at NPE2009 by the nearly 40 exhibitors offering bioplastics-related technology, including resins, specially designed additives, machinery, and processed goods, as well as the more than 50 conference presentations on the subject, according to the show’s blog reports. Interest in biopolymers has been growing among medical device manufacturers, especially in the past year, along with the green manufacturing trend and the desire to be more environmentally conscious, according to Johnson. “But existing products haven’t had the right performance characteristics until now,” he says.
PolyOne, an exhibitor at NPE, launched six new products at the show, two of which were biopolymers. Offering high impact performance, Resound biopolymer compounds are one of PolyOne’s product lines engineered to provide a functional biopolymer for the medical device market. Designed for durability, the compounds are suited for use in manufacturing medical device housings, most of which typically contain electronic components, according to Johnson. “These biopolymers have a higher heat resistance than past materials,” he explains. Resound compounds combine engineering thermoplastics with such biobased polymers as variations of polyhydroxybutyrate and biopolyesters. These compounds have the necessary heat resistance and processing capabilities to be used to make parts for IV pumps as well as respiratory, anesthesiology, and patient-monitoring equipment.
“Many manufacturers want to be able to say they have biopolymer content, but they need to maintain the same physical properties of the current materials they’re using in their applications,” Johnson says. In response to that need, PolyOne also created its Versaflex Bio thermoplastic elastomers (TPEs), which have 70% renewable content with no loss of such performance characteristics as heat resistance or clarity, according to the company. With enhanced performance and processing characteristics, TPEs are now being regarded by OEMs as functional materials in such applications as molded-in seals, gaskets, and living hinges, Johnson notes.
In Living Color
Biopolymers not only offer the sustainability that some medical device OEMs are seeking, some also offer the benefit of being compatible with material colorants. “There is an increased interest in masterbatches and UV additives for coloring products, and biopolymers are a good base polymer for those applications,” Johnson says.
While some niches, such as the catheter market, use colors for identification, there is also an emerging trend toward using color for company and product branding, as well as to appeal to end-users. To meet these demands, some suppliers, such as PolyOne, are making a point of incorporating colorability into the performance characteristics of its products. As part of its Geon product line, for example, PolyOne recently introduced new grades of metallic, precolored Geon vinyl compounds that are offered to help product designers improve aesthetics while eliminating environmentally unfriendly and costly painting options.
Similarly, The Dow Chemical Co. (Midland, MI; www.dow.com) offers custom and stock precolored options for its Calibre polycarbonate (PC) and Emerge PC/ABS blends, as well as other highly colorable polyethylene products. Its Health+ polymers, which were launched at NPE2009, also have the potential to accept some form of colorant. The increased interest in coloring options can be partly attributed to the increase in home healthcare and the changing way in which people interact with medical devices, suggests Jason Eckel, market development manager, rigid packaging and health and hygiene, for the North American basic plastics division of Dow. “Home healthcare is becoming more like other consumer markets,” he says. “People didn’t used to want others to know they had a glucose monitor, for example. But now they want them in a color they choose, like iPods or laptops,” he adds.
Fluid Delivery
While some market niches, such as glucose monitors, are pursuing enhanced aesthetics in materials, fluid-delivery applications continue to require improved functionality and antimicrobial features. “The driver behind the need for chemical resistance and antimicrobial features is the increased presence of infection in healthcare environments and Medicare and Medicaid’s decision last year to no longer provide reimbursement for hospital-acquired infections,” says Jill Martin, senior development specialist, North American basic plastics, Dow Chemical.
Most of the polymers in the Health+ platform are high-density polyethylenes that offer chemical resistance and can be blended with other polymers to enhance such performance characteristics, according to Martin. Not only do the polymers have the potential to accept colorants, but they also feature different melt index and density ranges for manufacturing such medical products as fluid-delivery kits, syringes, and breathing treatment and tubing systems. They also could potentially be used in medical device packaging.
|
Plastomer Technologies offers a high-purity PTFE material that can be used in valves, diaphragms, and pumps. |
Plastomer Technologies (Houston; www.plastomertech.com) also has recognized the continuing demand for polymers that can be used in such fluid-delivery applications as valves, diaphragms, and pumps. These products require a high level of purity and fluid-transfer cleanliness, explains Roxanne Dittrich, business manager for Plastomer Technologies.
The company recently introduced a high-purity polytetrafluoroethylene (PTFE) material, Texolon Style 8764HP, that offers chemical inertness and antistick properties. It is processed in a controlled environment to help eliminate contamination that is often found in PTFE moldings, Dittrich says. Offered only in pure, virgin PTFE form, the material provides wear and corrosion resistance necessary for critical medical and electronic applications.
“Plastomer realized there was a need for PTFE that would be processed and packaged for high-purity applications, such as those required in medical, electronics, or filtration, and developed this product to fill this requirement,” she says.
Notification of Change
Following such trends as colorability and sustainability can be a good business decision for both suppliers of materials and the OEMs who use them, but the slightest change to a material or component of a device can result in costly redesigns or testing for an OEM. Because of the potential redesign consequences, some suppliers are now increasing their efforts to keep OEMs informed of any changes or potential changes in formulation or availability of their materials.
“What we think is a minor change may be huge to a medical device OEM,” Eckel says. “They need the distinction between the original and new versions of a material to be known and [they need to understand] the impacts of those changes.”
In response to this need, Dow has launched a Notification of Change initiative for its products included in the Health+ polymer platform. The service is designed to help alleviate the stress on OEMs that are concerned with supply continuity, especially because raw material changes require recertification from FDA, says Eckel. “The Notification of Change guarantees a minimum notification regarding the discontinuation of a product or product composition changes.”.
For more information and articles on plastics and elastomers, go to www.devicelink.com/mpmn/materials
Copyright ©2009 Medical Product Manufacturing News
You May Also Like