January 1, 2006
REIMBURSEMENT
The reimbursement rules are changing for manufacturers. They may soon be more likely to seek coverage from private insurers than from Medicare or Medicaid.
By Barbara Grenell, Debbie Brandel, and John F. X. Lovett
|
Most manufacturers have some experience with the intricacies of coverage and reimbursement under Medicare. The rules for reimbursement are highly complex and subject to change. But at least reimbursement information for Medicare is readily available, and the process is standardized.
Even more challenging is the path to reimbursement from commercial payers (i.e., private insurers). Each commercial payer follows its own set of rules. Coverage and payment policies vary from payer to payer, and sometimes from product to product, for medical benefits including devices. Manufacturers must approach each payer separately, and there are no guarantees that an agreement will be reached. Success with one or more payers does provide some momentum and a better case with a subsequent payer, but there is no way to circumvent the one-by-one sell. The process of commercial reimbursement is both time-consuming and labor-intensive.
|
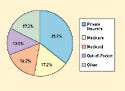
Figure 1. National health expenditures by source of funding, 2005. Source: CMS, Office of the Actuary. |
Nonetheless, commercial payers represent a critical market for almost all device manufacturers. According to the Centers for Medicare and Medicaid, private health insurers are the largest single source of funding for healthcare, accounting for more than $1 out of every $3 spent on healthcare in 2005 (see Figure 1). As more Medicare and Medicaid beneficiaries are encouraged (or required, in the case of Medicaid) to enroll in managed-care plans run by the commercial market, private insurers will begin to control an even larger piece of the pie.
Therefore, it is imperative that manufacturers learn and understand the coverage and reimbursement process used by private insurers. It is even more important to take it one step further and understand the nature of the private insurers' relationship with service providers. This includes knowing how those reimbursement policies affect physicians' and the hospitals' willingness to purchase a variety of medical devices. Armed with such knowledge, manufacturers are in a much better position to influence the process and achieve a more-positive outcome.
Insured and ASO Plans
|
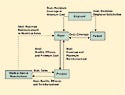
Figure 2. Conflicting goals in reimbursement and coverage relationships. |
The first thing to understand is that coverage and reimbursement vary not only by payer, but within each payer as well. Policies can vary by the type of plan (e.g., HMO, PPO, or indemnity) as well as by the specific employer covered by the payer. Private insurers generally offer two broad types of policies:
• Insured plans under which the employer pays the insurer a set monthly premium and the insurer assumes the risk for providing all covered benefits.
• Administrative services only (ASO) plans under which the employer retains the financial risk and contracts with the insurer to administer the plan. Large employers that are self-insured usually use ASO plans.
In an insured plan, an employer may opt to add certain benefits (known as riders) not covered in the standard benefit packages or it may simply offer one of the generic plans offered by the insurer. It may also offer options such as an HMO, which limits coverage to a network of providers, or a PPO, which provides out-of-network coverage, albeit at a reduced level. Each of these plans might have several options based on copay and deductible levels. Insurers have considerable leeway in designing these benefit plans and in developing and implementing policies with respect to both coverage and reimbursement.
In an ASO arrangement, an employer has considerably more influence over coverage and reimbursement decisions. Self-insured ASO plans are regulated by the U.S. Department of Labor under the Employee Retirement Income Security Act of 1974 (ERISA) statute. ERISA is a federal law that establishes minimum standards for private health plans to protect individuals who are enrolled in those plans. It requires the insurer to provide the participants with plan information. It also requires that there be a grievance and appeals process and gives the participants the right to sue for benefits or breach of fiduciary responsibility.
Insured plans are governed by state regulations that may impose requirements in terms of both coverage and benefits. For example, some states require that insured plans provide direct access to OB/GYN services, establish minimum stays for maternity cases, and require coverage of certain allied health professionals. While insured plans must meet these requirements, self-insured ASO plans do not have to abide by state requirements. Private employers dictate these decisions in the case of self-insured plans within the general guidelines established by ERISA.
Although each payer has its own process, there are many similarities across the industry. In most cases, a committee is charged with the responsibility for coverage policy. The committee is chaired by one of the corporate medical directors, and this committee is typically the first stop in the process of obtaining coverage. The request for coverage can be initiated by a provider, by a patient, or by a manufacturer.
At a minimum, payers require that the device have FDA approval (either via a 510(k) or premarket approval application) before even considering a review. In addition, they look for unbiased clinical literature that documents the safety and the efficacy of the product. Most payers use an external assessment company (Hayes, ECRI, or the Blue Cross Blue Shield Technology Evaluation Center, for example) to review the literature and provide the payer with a recommendation. The payer may supplement the information with its own literature review, or it may use outside clinical consultants to review the data.
Physician advocacy is an extremely important component of the coverage decision-making process. The more physicians who indicate an interest and belief in the product, the stronger the case will be. If the manufacturer initiates a coverage review, it is important to identify a group of supporting physicians who are willing to champion the product to a payer. The physicians should be participants in the payer's network and ideally should be viewed as leaders within the community.
After reviewing all of the documentation, the committee makes a final determination. Although the time frame varies, most decisions are made within six months. Depending on the circumstances, if the provider has initiated the request on behalf of a patient, the payer may make an interim, case-specific determination so that payment is allowed before a final decision is made. These situations, however, are rare. They generally occur only if the patient has no alternative and it is already likely that the committee will recommend approval.
Once a decision to cover the device is made, there are still a number of hurdles to overcome before payment. The payer must address internal implementation issues, including the following:
• Reimbursement methodology and payment level.
• Appropriate coding.
• Adjustment of the claims processing system to accommodate the new device.
• Evaluation of the need for use-management controls or quality assurance guidelines specific to the device.
These decisions are made by entirely separate departments within the company and require ongoing education of key staff.
If the payer decides to deny coverage, there is generally an appeals process within each state. A patient or his or her designee can appeal to that state's department of insurance if there has been adverse determination. The specific processes and time frames vary by state and, of course, only apply to insured plans covered by the state. Self-insured plans are covered by the ERISA statute and must follow federal guidelines in establishing the appeals process.
Reimbursement
Once coverage issues have been resolved, the second-greatest challenge for a device manufacturer is reimbursement. The initial reimbursement level may be based on payment levels for similar devices or technologies. If Medicare has already approved the device, the payer may peg initial reimbursement at some percentage of Medicare. The availability and level of reimbursement is one of the most important factors that will affect sales of any medical device. The complexity of the relationships of the participants and their conflicting goals (see Figure 2) makes reimbursement one of the most difficult parts of the process.
From the manufacturer's perspective, the goal is to maximize sales. To do so, however, a provider must be convinced not only of the efficacy and quality of the product, but also of the potential for reimbursement. The manufacturer has the greatest vested interest in maximizing reimbursement. Yet the manufacturer is the furthest removed from the process and, in fact, often has only limited relationships with potential payers. Payers and providers, however, have close ties and negotiate reimbursement levels as part of their contractual agreement.
Reimbursement for medical devices that are used as part of a surgical procedure (inpatient or outpatient) is subject to negotiation and varies substantially by provider and by payer. For example, a hospital may be reimbursed for a particular type of procedure on the basis of a case rate. In this situation, the hospital receives an inclusive payment for the procedure, which often accounts for the cost of devices and implants. Some hospitals are successful at negotiating separate payments for high-cost devices or implants (generally at the invoice cost); however, this is not always the case. Payment for high-cost new technologies may be based on exceptions for some period of time. Ultimately, payers will look to lump the cost within the overall payment rate. If the basis for payment is a per diem rate, the hospital is more likely to be reimbursed specifically for the device. Typically, reimbursement for an implant is based on the invoice cost.
If there is no separate payment, high-cost devices could become an issue for the finance department in the hospital. The decision regarding which medical devices and other supplies to order is generally influenced by the physicians. They will let the purchasing department know their preferences, and, as long as no red flags are raised, the process will generally go unimpeded. If the finance department begins to see that the cost of a device is not being recouped through the reimbursement process, it may intervene, and sales will ultimately be affected.
However, waiting for a hospital to unearth such a problem, which can take anywhere from six months to a year, is a poor way to do business. It is important to work with these institutions to manage and resolve reimbursement issues.
For devices and other products that are reimbursed separately, sales will depend on the reimbursement level, the ease of administration, and the opportunity for physicians to realize an additional revenue stream. It is extremely important that manufacturers provide payers with enough information so that the payment policy reflects the actual cost of the device.
Targeting Payers, Getting Coverage
Given all these complexities, what can a manufacturer do to influence the process and achieve the best possible outcome? First, it is never too soon to begin thinking about issues related to coverage and reimbursement. Decisions regarding pricing, modality, marketing, and distribution should all be made after considering the effect that alternative technologies may have on reimbursement and coverage. There is little to be gained by launching a product that has limited likelihood of being covered or reimbursed at the appropriate level.
Second, it is imperative that manufacturers understand how products will be reimbursed, who is involved in determining reimbursement, and who makes the purchasing decision. Knowing who is involved at each point and what their motivations are is essential to targeting resources effectively.
The next step is to prioritize the markets based on the demographics of the product. It is impossible for most manufacturers to approach all payers simultaneously; therefore, it is most effective to develop a targeted approach. Select those payers that match the product's demographics. Targeted payers should have a large base, and they should be likely to influence other payers, such as national plans and local Blue Cross plans. Establishing relationships with private payers is almost as important as getting to know physicians. Developing these relationships enables manufacturers to facilitate the decision-making process and ensure that they will have the opportunity to present their case.
Lastly, it is important to present the facts that a payer is most interested in hearing. Payers are concerned about three main issues: cost, safety, and efficacy. Insurers are at risk for the overall medical costs of their members. Therefore, even a device that is more costly on a unit-cost basis may get coverage if it reduces overall healthcare expenditures. Most payers understand that new technology is initially more costly. Manufacturers must demonstrate the value of those costs. If a payer is convinced that the product is as efficacious and safe as an existing product or procedure, then the manufacturer will have an excellent chance of obtaining reimbursement. Bringing along a physician advocate is particularly helpful when meeting with a payer's medical director. Manufacturers must be prepared with all the facts the first time around. Understanding the process and interacting with commercial payers can pay off in the long run.
Barbara Grenell is founder and president of Preferred Heath Strategies (Rye Brook, NY) and can be contacted at [email protected]. Debbie Brandel and John F. X. Lovett are principals with Preferred Health Strategies. They can be contacted at [email protected] and [email protected], respectively.
Copyright ©2006 Medical Device & Diagnostic Industry
You May Also Like