April 1, 1999
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
An MD&DI April 1999 Column
From total laboratory automation to personal healthcare monitors, the application of advanced technologies is rapidly creating a new generation of IVDs.
In the sector of the medical device industry devoted to the design and manufacturing of in vitro diagnostics (IVDs), a quiet revolution is taking place. In the near future, say industry observers, the application of advanced technologies will lead not only to wholly new products for the diagnosis of human disease, but also to changes in how, where, and by whom such diagnostic products are used.
Advancement of the field is being forwarded by a variety of factors, not all of which can be untangled from one another. "This is a 'chicken or egg' situation," says Al Snitkof, chief technology officer at Hemadyne Research Inc. (White Plains, NY). "It is difficult to say whether marketplace trends have turned into technological trends or whether technology is driving the marketplace for IVDs.
 Photo courtesy of Dade Behring Inc. (Deerfield, IL).
Photo courtesy of Dade Behring Inc. (Deerfield, IL).
"Certainly, the expectations of IVD users translate into requirements for IVD designers," he adds. "But at the same time, advances in technology are giving IVD designers spectacular new abilities to anticipate the future requirements of end-users and to provide new and enhanced levels of functionality at the time they are demanded by the marketplace."
As an example, Snitkof points to the technological advances brought about in the semiconductor industry, where the rapidly decreasing cost of producing complex application-specific integrated circuits has been matched by significant increases in the density of transistors per square millimeter of chip. "Adopting such technologies enables IVD developers to achieve levels of functionality unimaginable even a year ago," he says. "The result is an improved ability to create new IVD products that meet customer requirements more quickly than ever."
BUSINESS PRESSURES
Whatever confluence of forces may be driving advancement in the IVD industry, one factor can't be ignored. "The most significant marketplace factor influencing the development of new IVDs is cost," says Tom Tsakeris, president of Devices and Diagnostics Consulting Group (Rockville, MD). "Buyers are demanding tests that are easy to use and give rapid results, and they are unwilling to compromise accuracy or reliability. All of these characteristics have to be delivered at the lowest possible cost."
During the past decade, the IVD sector has come under increasing pressure to contain costs. Health maintenance organizations have gradually reduced the amounts that they are willing to pay for laboratory tests and have pressured clinical laboratories to reduce their charges. In turn, manufacturers have also felt the pinch.
For many IVD companies, the challenge of reducing costs has led to products designed to minimize the labor involved in performing diagnostic tests. "Saving on labor costs can be achieved by designing tests to be performed by individuals with less training who are also paid less, replacing the work of a doctor or nurse with that of a ward clerk or medical technician," says Joanne Stephenson, director of business development for Response Biomedical Corp. (Vancouver, BC, Canada). "But to meet this objective, the technologies must be especially simple to use—essentially foolproof—so that valid results can be obtained consistently from an operator with little or no training."
Regulatory requirements also determine the amount of labor associated with diagnostic testing. "To meet the requirements of the Clinical Laboratory Improvement Amendments of 1988 [CLIA], most labs still need to validate test performance and quality control," says Tsakeris. "So the less work that a test requires to fulfill this function, the more marketable it will be. This is one of the reasons that the market for point-of-care [POC] devices is becoming so large, despite an ongoing debate over whether such products result in cost savings."
For Stephenson, the cost savings brought about by POC devices are not at all debatable. "Anything that saves time saves money," she says. "Products need to be cost-effective in the big picture. Those that can give immediate results as close to the patient as possible will hasten treatment and therapeutic decision making and will ultimately reduce costs by preventing additional visits to doctors' offices, longer hospital stays, additional lab tests, and so on."
All of these efforts to control costs have inevitably had an effect on the types of diagnostic products being designed to fill the pipeline. "Cost-containment pressures have begun to divide the market into two dominating sectors—automation and near-patient care," says Stephenson.
AUTOMATION
According to Katie Smith, director of clinical affairs at Gen-Probe Inc. (San Diego), the single trend that is having the greatest effect on the design of new diagnostics can be described in three words: "Automation, automation, automation." But automation means different things to different manufacturers.
 Figure 1. The Architect TLA system by Abbott Diagnostics (Abbott Park, IL) can be configured to incorporate multiple immunoassays or to combine immunoassay and clinical chemistry analyzers. Shown here, the i2000 immunoassay analyzer (launched in January) combined with the c8000 clinical chemistry analyzer (scheduled for launch later this year).
Figure 1. The Architect TLA system by Abbott Diagnostics (Abbott Park, IL) can be configured to incorporate multiple immunoassays or to combine immunoassay and clinical chemistry analyzers. Shown here, the i2000 immunoassay analyzer (launched in January) combined with the c8000 clinical chemistry analyzer (scheduled for launch later this year).
At its grandest, automation means total laboratory automation (TLA), an approach that makes use of large-scale, integrated systems to automatically carry out all of the routine testing (and even some fairly esoteric testing) performed in a clinical laboratory. Planned modules of the Architect system by Abbott Diagnostics (Abbott Park, IL), for instance, can perform both clinical chemistry and immunochemistry tests (Figure 1). The CLAS by Roche Laboratory Systems (Indianapolis) can incorporate modules for clinical chemistry, hematology, coagulation, and urinalysis testing.
Automation Type | 1997–1998 (%) | 1998–1999 (%) |
Integrated systems | 40 | 41 |
Centrifugation | 37 | 37 |
Aliquoting | 31 | 35 |
Storage | 24 | 28 |
Retrieval | 24 | 23 |
Coverslipper | 22 | 21 |
Pipetting | 21 | 21 |
Staining | 19 | 20 |
Transport | 18 | 17 |
Total laboratory | 13 | 12 |
Table I. Percentage of respondents that expect named types of laboratory automation to be implemented in their clinical laboratories within the next five years, according to surveys conducted in 1997 and 1998 by the Clinical Laboratory Management Association.
Depending on the type and size of its instrumentation, such a system can cost upward of $3 million—a daunting amount even for the largest clinical laboratories. Such a large price tag may be one reason that clinical laboratories have been slow to adopt the TLA approach. A 1998 survey of senior-level lab managers conducted by the Clinical Laboratory Management Association (CLMA; Wayne, PA) revealed that only 12% of laboratories expect to implement TLA in the next five years—the lowest percentage for any of the automation strategies included in the survey (Table I).
What makes TLA systems competitive is the fact that they can eliminate much of the labor involved in lab testing. According to this line of thinking, says Stephenson, "the greater the automation of a lab, the more it can save on labor costs."
But adoption of the TLA model also has significant implications for the structure of clinical laboratories. Because only those laboratories that perform the greatest number of tests can afford to support large, complex, and expensive TLA systems, the TLA approach inevitably leads to greater centralization of laboratory testing—another trend revealed by the CLMA survey. The survey results suggest that restructuring among clinical laboratories could soon result in the demise of self-standing and independent clinical laboratories. More than a third of the respondents (37.4%) said that their laboratory had joined a network or alliance in the previous 12 months, and nearly a third (29.3%) indicated that they would be doing so in the next 12 months.
Whatever its advantages, the TLA approach isn't for every laboratory—and certainly not for every manufacturer. The vast majority of instruments on the market take a less-integrated approach to automation. Some instruments include specialized components devoted to sample transport, storage, and retrieval. Others automate fluid-handling steps such as pipetting, aliquoting, and centrifugation. A few integrate some or all of these features into a self-standing automated module that resembles one portion of a TLA system.
What many of these automated analyzers have in common is their relative simplicity of operation, which can make them suitable for use in a variety of settings. "The products with the greatest appeal are those that reduce labor, those that are simple to use (and therefore simple to sell), and those that are truly platforms or can be integrated easily with existing technologies," says Stephenson.
Automated features play a large role in enhancing the marketability of all such instruments, whatever their size and complexity. But not every manufacturer takes the same approach to automating the various functions that their instruments perform. Following is a summary of some of the operations most commonly automated, and how some manufacturers are making their instruments perform those operations.
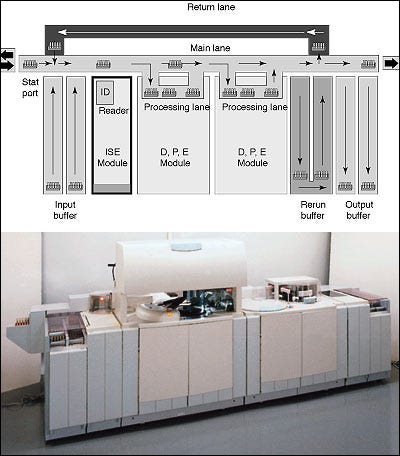
Figure 2. The bidirectional track of the Modular TLA system by Roche (Indianapolis) speeds throughput by routing samples to the first available analyzer.
Sample Transport. From the time that a patient sample is drawn to the time that it is placed in refrigerated storage, some method of transport must be provided. TLA systems don't attempt to eliminate the need for laboratory couriers, but they do offer an efficient means of moving sample tubes from test to test once they have been delivered to the lab. Generally speaking, TLA systems employ conveyers to transport sample tubes, which are usually placed in a small rack or cassette. Some systems configure the transport conveyor like a racetrack, around which sample tubes progress in order to reach each of the system's analyzers. The Modular TLA platform by Roche uses a bidirectional track to route samples to the first available analyzer, and an open processing lane within each of the system's modules enables the operator to assign stat priority to a particular sample (Figure 2).

Figure 3. The Aeroset clinical chemistry analyzer by Abbott Diagnostics (Abbott Park, IL) uses 2-D bar coding, which can carry up to six times as much information as traditional bar coding.
To keep track of the movement of the sample tubes, TLA systems employ bar code readers that scan each tube and match it with the testing instructions stored in the system's computer. Coded instructions can be used to tell the system where to send each sample tube and what tests to run when it gets there. The bar code reader used in Abbott's Architect system supports Code 39, UPC/EA coding, and ASTM Code 128. Because of the limited space available on sample test tubes, diagnostic firms have been among the first device manufacturers to adopt two-dimensional bar coding, which can carry six times as much information as traditional bar codes. The newer 2-D coding is used on Abbott's Aeroset clinical chemistry analyzer (Figure 3) and will also be incorporated in the company's Architect c8000 clinical chemistry analyzer, scheduled for launch later this year.
Although automated sample transport is a feature that generally appears only in TLA systems, stand-alone analyzers may also use racks for holding sample tubes, as well as bar coded instructions for running tests. On such instruments, transport is usually limited to the movement necessary to place the sample tube into the machine for analysis, and to withdraw it into a holding area when the test has been completed.
In all such automated transport systems, manufacturers are making extensive use of the power of computers, robotics, and bar coding technologies. Add to that sophisticated motors and motion controls and one begins to have an idea of the complexity associated with sample transport in today's IVD instruments.
Unattended Operation. The goal of reducing labor costs has significant implications for diagnostic analyzers and their features. Manufacturers of automated systems are quick to point out the "walkaway time" of their products, a key indicator of the sophistication of a product's software systems, as well as its capacity to store reagents, buffers, and waste materials. The Architect i2000 system by Abbott, for instance, boasts a five-hour walkaway time, with enough buffer to run 1000 tests and dry waste storage for 1000 reaction vessels. The Dimension RxL by Dade Behring (Deerfield, IL) features onboard tracking of reagents and other consumables and automatically prompts the operator when more supplies are needed.
A related characteristic of automated diagnostic analyzers is simplicity of use, a key factor in maximizing the marketability of such systems. Under the CLIA regulation, tests that have been granted waived status can be performed by virtually anyone, while those assigned a moderate- or high-complexity rating can be performed only by specially qualified personnel. Manufacturers are therefore working hard to make their instruments as simple to use as possible, starting with the computer-driven user interfaces that enable laboratorians to tell the systems what to do. Automated quality control and calibration are also common features of such systems, permitting them to be operated in settings other than clinical laboratories and outside the direct control of highly trained laboratorians.
Sample Preparation and Testing. When traditional testing methods or semiautomatic systems are used, some assays require significant sample preparation before the actual clinical tests can be performed. Necessary preanalytical steps can include bar code labeling, centrifugation, decapping, aliquoting, titration, dilution, and recapping and can be the most labor-intensive and time-consuming phase of testing. Diagnostic manufacturers are finding ways to eliminate such delays by integrating the needed functions into their analyzers or by redesigning the tests to eliminate steps. The Modular TLA system by Roche, for instance, can be configured with a unit that performs centrifugation, aliquoting, bar code labeling, and sorting. The Stratus CS cardiac marker analyzer by Dade Behring takes another approach, using closed-tube sampling to eliminate decapping and recapping and using premeasured reagent cartridges to eliminate the need for user mixing.
The menu of tests that can be performed by an automated system is an important element of its marketability. The Architect i2000 system by Abbott, for instance, was launched in January with an initial menu of 26 tests, with another 36 promised by 2001 and a further 26 targeted for future appearance. The BM/Hitachi 747 by Roche has a resident test capacity of 35 tests, which can be selected from a total menu of 65 applications approved for the analyzer. And the Dimension RxL by Dade Behring can accommodate 48 tests, selected from a menu of more than 60.

Figure 4. The Dimension by RxL by Dade Behring (Deerfield, IL) can house up to 88 reagent cartridges in two carousels.
Systems with such broad test capabilities require an equivalently large number of onboard reagents. With the addition of an inventory management system module, Dade Behring's Dimension RxL can be configured to house as many as 88 reagents on board (Figure 4), while Abbott's Aeroset includes 56 onboard reagents. Because the reagents approved for one company's analyzer are typically not suited for use in another company's equipment, the testing and reagent menus offered by manufacturers are often the keys to selling the system as a whole. Although laboratory users might prefer instrument platforms that can make use of reagents from a variety of sources, manufacturers of large automated systems have so far resisted such interchangeability. Instead, their sales approach has focused on convincing lab purchasers of the advantages of choosing a single company's platforms and reagents for all analytical purposes.
Throughput. Of all the measures of automated equipment, high throughput is probably the single characteristic most promoted by manufacturers. And with high-volume laboratories as the target market, such a trend seems unlikely to cease anytime soon. "Manufacturers are making more use of robotics to increase lab test throughput," says Katie Smith.
Among the immunoassay analyzers now on the market, Abbott's Architect i2000 boasts throughput of 200 tests per hour, a rate that can be increased to 800 tests per hour by linking additional systems to the original unit. Dade Behring's Dimension RxL can perform 167 immunoassays per hour or a total of 788 chemistry and immunochemistry tests per hour. Meanwhile, for clinical chemistry testing, as many as six Modular analyzers by Roche can be linked together to provide throughput of 10,000 tests per hour.
Despite manufacturer emphasis, however, throughput isn't always the best measure of an analyzer's output. The TLA systems now on the market acknowledge this in their subsystems devoted to stat and reflex testing, which offer users the ability to push a particular test ahead of others in the queue or predetermine a sequence of follow-up tests based on the result of an initial assay.
Since high throughput comes with a high price tag, manufacturers of TLA systems are offering their systems as modules that can be scaled to fit the needs of laboratory purchasers. Both the Architect i2000 by Abbott and the Modular system by Roche can be scaled up incrementally as a laboratory's volume increases, enabling these manufacturers to lock in their customers for future growth.
Electronic Interfaces. Many of the automated systems now on the market are taking advantage of information-age technologies to offer features unthinkable a few years ago. Abbott's Architect system, for instance, includes a module for computer- based training, an on-line manual, and modem-based system diagnostics. Dade Behring's Dimension RxL also offers modem-based diagnostics, and optional features include a quality assurance program that compares QC data against an existing database and bidirectional electronic links to the user's laboratory information system.
In the world of in vitro diagnostics, methods of clinical testing are often known by the key substrates on which they are performed. Thus, culture tests are associated with the ubiquitous petri dish; immunoassays have become familiar in the form of membrane-based tests; and, if a host of competing companies have their way, molecular diagnostics will soon become forever linked to the platform called a DNA chip. The essential substrate of a DNA chip is usually a glass or plastic wafer that has been specially modified to form receptacles for genetic probes. The wafers are produced using nano- and microscale fabrication techniques originally pioneered in semiconductor manufacturing, including microphotolithography, laser cutting and etching, and substrate bonding. Onto this prepared substrate are bound genetic probes, single strands of DNA that serve as capture agents for their complementary strands if they are present in a patient sample.
The most common format for the placement of genetic probes on a DNA chip is an array, which can be fabricated to contain as many as 20,000 elements using today's manufacturing techniques. But in their most elementary form such arrays are essentially static; they cannot perform the preanalytical steps necessary to prepare genetic material for testing, and the results of such testing cannot be easily analyzed. DNA-chip manufacturers are working in earnest to overcome these limitations, using microfluidic technologies to develop so-called "lab-on-a-chip" formats and designing analyzers that can readily interpret and display test results. According to many analysts, creation of such integrated chips will clear the way for molecular technologies to finally realize their potential in the diagnostic marketplace. One final obstacle that will have to be overcome, however, is the need to manufacture such DNA chips in compliance with the good manufacturing practices requirements of FDA's quality system regulation. Until now, with most of their products designated for use in research laboratories, most chip manufacturers have paid little attention to the type of fabrication necessary to do business in a regulated industry. But with the commercial potential of a waiting diagnostics industry before them, it's a fair certainty that chip manufacturers will soon be laboring to clear this final hurdle. |
NEAR-PATIENT TESTING
For all the excitement generated by large automated diagnostic systems, most manufacturers are finding their market elsewhere. "There is a trend away from the high-volume, centralized laboratory instrumentation traditionally offered by such large healthcare corporations as Abbott and Roche," says David Carville, vice president for new product development at Array Medical (South Bend, IN).
"Many smaller companies such as Biosite, Biostar, Luminex, Quidel, and Response Biomedical are actively pursuing near-patient and point-of-care testing in all areas of the IVD market," he adds. "The precision of such tests is gradually approaching levels previously attainable only by larger analyzers in a centralized laboratory, with the coefficient of variance for many tests now at lab levels of less than 5%. Such precision is paving the way for virtually all IVD testing to be performed on a stat basis—that is, at the patient's bedside instead of in a central laboratory. In the future, only specialized tests will be performed in a central laboratory setting."
About the Author(s)
You May Also Like