June 1, 2009
The Future
|
Image courtesy of iSTOCKPHOTO. |
Design Trends: A More Personal Approach
From my vantage point as an industrial designer working to improve the usability and appeal of many different kinds of medical devices, I observe some product personalization trends that I believe may lead to better healthcare experiences for all.
|
The Stork ultrasound is an example of how medical devices are becoming more patient-centric. Image courtesy of BRIDGE DESIGN INC. |
Some of what makes for great consumer products is finding its way into medical products, especially those trends that are very patient-centric in use. This is particularly true of products designed for a specific group of users. Having a focus frees designers from the frightening notion that a device must be all things to all users. It also allows them to develop products that result in better patient experiences. An example of this trend is “Stork,” a conceptual portable ultrasound that we designed targeting the prenatal market. This mother-centric device focuses on making the ultrasound experience pleasant and hassle-free and provides features not yet on the market: a second display so that the mother can comfortably share the view and the ability to directly e-mail images to family and friends. Unlike the average ultrasound machine, the Stork is nonthreatening, even playful in its appearance. Expect to see much more of this kind of design thinking in the next 5–10 years.
|
The Stork ultrasound in use. Image courtesy of BRIDGE DESIGN INC. |
Another development that is making headway is the combination of imaging advances and powerful 3-D virtualization techniques (thanks to chips and software spun off from the gaming industry) to create new ways of guiding surgeons and interventionists to their quarry. This presents usability challenges because the virtual and real worlds have to somehow be brought into alignment: if you are not sure where you are starting from or where the horizon is, how do you navigate? Such interfaces are in their infancy and will ultimately benefit from the power of 3-D immersive visualization techniques, perhaps combined with interesting new tactile feedback. Companies like SuperDimension (which has commercialized this technology for lung biopsies beyond the reach of bronchoscopic visualization) point the way. It's likely this technology will show up in other hard-to-reach areas, such as deep into the brain vasculature.
Sidebar: |
We will also see a new twist on the electronic medical record (EMR). Current EMRs are sketchy at best, and there are real issues with lack of standards. But fast-forward 10 years, and we will see much smarter devices that act on patients according to what they know about the person's medical history as well as what the sensors are reading. A vital-signs monitor might warn a nurse about some unusual patterns that, combined with previous history, warrant different treatment. An insulin pump connected wirelessly to both continuous monitoring equipment and recent lab results could tailor advice on what to eat and how to bolus for better blood glucose management.
Factor all of this personalization in with the advances in genetic profiling à la 23andMe (a genomics and biotechnology company), and now all we have to do is sort out the privacy and insurance “preexisting condition” issues. And you used to think FDA approval was the problem.
Imagining the Future of Medical Devices
Wayne Rogers, Consultant
If the past is a prologue to the future, innovation will be further restricted. In the past 30 years, innovation was wide open, like the West of old was to the nation, but increasing costs, limiting accessibility, regulations, and other restrictions will dampen unlimited innovations seen in the past.
|
The da Vinci robotic surgical systems are paving the way for the future of robotics in operating rooms. Image courtesy of INTUITIVE SURGICAL |
The innovation that does continue will do so because baby boomers will increasingly stay in the workforce—some well into their 80s. Unlike past generations, their ideas of retirement will include working, so that they will have the resources to purchase new innovations that generations past could not.
Robotics is one of the developments that will survive and evolve to play a stronger role in medical devices. Limbs and body parts will be increasingly replaced with robotics.
|
In addition to imaging inside the body, a pill-sized robot is being designed to deliver drugs to targeted areas. Image courtesy of CARNEGIE MELLON UNIVERSITY |
Also, miniaturization will continue to such an extent that medical devices will be based on nanotechnology: some devices will be swallowed or put in the bloodstream or will even be implanted intracutaneously. The size of a pill drug and a medical device will be similar.
Active electrical- and electrical-chemical–driven devices will increase in the future, as part of miniaturization, but also as part of remote control, tracking, and continuous monitoring. Because radiation harms active electrical devices, these advances will require processing methods besides traditional radiation.
Steam sterilization—the workhorse in healthcare facilities—will be replaced by more heat- and nonhumid-tolerant processing. New methods will be required for sterilization of active electrical components that would be damaged if sterilized with irradiation methods.
A Tough Regulatory Road May Be Ahead
Larry Pilot, Attorney at Law
As for the next 30 years, there is much to be gained from examining the successes and failures associated with management of the 1976 and subsequent amendments to the Federal Food, Drug, and Cosmetic Act (FD&C Act). If a responsible pause is not exercised now by Congress and the administration, it is likely that the regulation of the device industry and the contributing research-oriented healthcare community will becomeincreasingly oppressive and discouraging to the type of entrepreneurs who have created what had been an inspired and largely self-regulating industry.
|
The Servo-i NAVA, a neurally adjusted ventilator assist device, detects respiratory signals sent from the brain to the diaphragm and transmits those signals to the Servo-i ventilator. Using the electrical activity of the diaphragm, NAVA represents a paradigm shift away from conventional mechanical ventilators. Image courtesy of MAQUET INC. |
As an FDA “enforcer” for nearly 10 years, I frequently expressed that wrongdoing likely would be discovered and resolved through the efforts of the plaintiffs bar instead of through FDA. It requires the application of vast resources for the agency to identify a particular problem among the hundreds of thousands of device types manufactured by thousands of different companies.
Any start-up or existing device manufacturer has more to fear from marketing an unsafe or ineffective device—whether or not approved or cleared by FDA—than possible remedial initiatives undertaken by FDA. In this context, it will be unfortunate if Congress or the administration alters the Supreme Court preemption authority contained in the 1976 Medical Device Amendments. The states have the opportunity now to seek exemption from preemption by petition to FDA. This approach is much more sensible to application of states' rights than the alternative, which could result in individual juries overruling the approval or clearance decisions made by qualified FDA personnel.
If past is prologue and Congress tampers unwisely through questionable legislative initiatives, I expect that the regulatory environment will become more burdensome and disheartening to the innovators who have contributed to the advancement of medical device technology.
Thinking Small in the Future
Andrew Dallas, President and CTO, Full Spectrum Software
Nanotechnology will play a huge role in the future of medicine, so I will start by thinking small. There will be a convergence of genomics and nanotechnology. In fact, it is entirely possible that we may move beyond nanotechnology to pico or femto technology. Since both of these technologies are in their infancy, 30 years from now, we should be able to understand the mechanisms of action of virtually all known pathologies and how to deliver the perfectly structured, scaffolded, and packaged RNA interference (RNAi)—or even merely a subset of the RNAi—to the site of the pathology using an intelligent nanobot. With nanomaterials a reality and with nanomachines already mimicking flagellated bacteria to act as motors, it's easy to imagine specialized machines that can target malignant or diseased cells. It's also easy to envision our understanding of the entire human body beyond its molecular and genetic structure, down to its atomic and even subatomic structure and the physics that define the laws at those levels.
|
The VeinViewer is the first and only patented technology to locate subcutaneous veins and project real-time images of their location onto the surface of the skin. Clinicians can visualize the target area and observe any movement of the vasculature. Image courtesy of LUMINETX |
Further, nanobots or picobots may be the primary tool for discovery of diseases states, constantly monitoring all human vital signs and probing for various pathologies on a regular, preprogrammed basis. How the nanobots are programmed will be the result of a complete scan of our entire genome and the likely and perhaps known disease states that we will develop at a certain age. What we call “predictive medicine” today may become 100% accurate diagnostic medicine in the future.
|
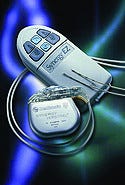
The Synergy EZ neurostimulation device represents the future of device design. Image courtesy of MEDTRONIC INC. |
One might also imagine generalized machines intelligent enough to know when they need “charging” with a particular nanite, customized RNAi, or pharmacological agent. These machines would have the ability to alert the patient and to schedule a doctor's visit for the required agent.
Prosthesis technology will evolve to a point where even the most complex organs such as eyes, lungs, and neurological structures will be replaced with the regrowth of perfectly matched, genetically identical organs. Stem cell research and a profound understanding of cell genesis based on advanced genomics and proteonomics may form the basis of creating genetically identical organs. Allografts will be rendered obsolete as nanobots deliver the required materials to stimulate, monitor, and direct the natural regrowth of a patient's organ.
Patient diagnostics will come to your home or, more likely, to your PDA. The PDA technology 30 years from now will perform all diagnostics and have the information delivered electronically to your physician. The role of physicians will change dramatically. They will evolve from their current role as physical diagnosticians to highly advanced programmers and biomedical engineers.
Lifetime health records will likely be implanted in a nanochip in the patient's body. Just as we implant dog identification chips, these advanced nanochips will be capable of storing a lifetime of medical data in exquisite detail. If you suffer a trauma, rescue workers would scan your chip and transmit your entire health record, genetic makeup, and personalized nanobot activities directly to the waiting physician.
Whatever the future holds, it is certainly going to reshape the medical device industry and the role of device manufacturers. Devices will become a more important, more integral part of all aspects of healthcare from the bedside to the surgical suite.
Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like