May 1, 1999

New Treatment for Obstructive Sleep Apnea Employs Radio-Frequency Energy
Treatment Made Possible by Device Constructed of Innovative Plastic
By Karim Marouf, MPMN Managing Editor
Habitual snoring, obstructive sleep apnea, and other upper-airway disturbances affect millions of people. Whereas existing therapies using lasers or traditional surgical techniques to remove tissue can be expensive, painful, and debilitating to the patient, a minimally invasive procedure developed by Somnus Medical Technologies Inc. (Sunnyvale, CA) uses radio-frequency thermal ablation to treat the disorder.
To perform the procedure, the physician uses a tiny electrode on the Somnoplasty instrument to transmit low levels of radio-frequency energy to create molecular friction in subsurface tissue. Within a few weeks, the treated tissue is resorbed by the body, while soft tissue is tightened and the size of the structure, typically the uvula, is decreased. Perceived levels of snoring are then significantly reduced.
Designing the instrument was a challenge taken on by a Somnus design team led by Ben Nordell. The device was to consist of a disposable catheter-based tip, made of ABS, and a reusable main body, which had to be made of a plastic that could withstand repeated sterilization. Since the handheld device would have to survive being dropped by a physician, the plastic used for the body had to be impact resistant. Certainly many plastics can provide this feature, but it was a challenge to find one that would maintain its strength after repeated sterilization cycles.
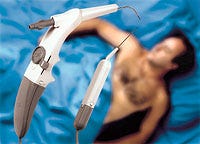 The main body of the Somnoplasty instrument was made using Radel R, a plastic that retains its impact resistance after repeated steam sterilization.
The main body of the Somnoplasty instrument was made using Radel R, a plastic that retains its impact resistance after repeated steam sterilization.
The design team first consulted a study group consisting of several doctors who specialized in the field of sleep disorders. The team learned that the device would usually undergo steam sterilization. Since boiler additives such as morpholine, used to stop corrosion in the steam sterilization system, can degrade plastics, the device needed to be made from a chemically resistant material.
Making the device out of stainless steel, which would have been sturdy and sterilizable but also heavy and expensive, was never seriously considered. Explains Nordell, "With these new engineering plastics, you can do a great deal that you couldn't have before, so it is no longer necessary to use."
The device designers looked into various plastics, including several from Amoco Polymers Inc. (Alpharetta, GA), part of the BP Amoco Group. One that stood out above the rest was Radel R polyphenylsulfone. "Radel R is suitable for any device that sees repeated sterilization and needs to maintain its impact strength," explains Daryl Brace, public relations manager for Amoco. "Many polymers become brittle, but Radel R has excellent hydrolytic stability, so it can withstand numerous steam sterilization cycles and still maintain its physical properties." Previous applications for Radel R have included surgical instrument trays and laboratory animal cages.
Radel R thus fit the design team's criteria for impact strength, sterilizability, and chemical resistance. A less critical, but still important feature, was that since surgeons are accustomed to using high-quality stainless-steel equipment, the material had to both look and feel right to them. Stephen Rudy, exvice president of marketing for Somnus, says, "We would repeatedly go out and hand materials to surgeons and ask, 'How does this feel?' Any materials that they didn't like got knocked off the list." Nordell affirms, "We wanted something with a sturdy feel to it. It was very important to project that impression."
It will be no surprise to design engineers that one of the greatest challenges that Somnus's team had to overcome was pressure to develop the device quickly. The project from start-up to molding the parts took less than a year. "There were a lot of confining issues that we had to work through," says Nordell. One was designing a simple but very durable and resilient latch mechanism between the disposable and reusable parts. A snap fit was the solution.
Another challenge was creating prototypes to analyze the appropriateness of the material. Nordell says, "We didn't have a whole lot of money to spend on prototype tooling. To prove out the concept and verify its sterilizability, we purchased these materials and did 3-D machining on all the parts prior to even releasing tooling." Here the team discovered another benefit of Radel R--that it is easy to machine.
Molding Radel R did present some challenges. "The flow characteristics and the setup parameters necessary to get a flawless part were a tough chase," says Nordell. "But we worked with Amoco very closely, and they provided the technical expertise to our molder to dictate how the material should be run, and eventually it worked very well."
Brace emphasizes that Amoco specializes in working closely with both its clients and its clients' molders. "The whole idea of providing a package of services is that we help from design to finished part. We provide advice on tool design, how to process the polymer, how to detail the component, what kind of radiuses to put in, and how to maximize impact resistance."
The finished device is the first of its kind approved by FDA to treat sleep apnea. Because of it, a lot of people will sleep a little easier, and the designers at Somnus can be proud of that accomplishment.
MPMN is seeking success stories like this. If your company has one to share, please contact managing editor Karim Marouf at 11444 W. Olympic, Ste. 900, Los Angeles, CA 90064-1549; 310/445-4200 or e-mail [email protected].
Copyright ©1999 Medical Product Manufacturing News
You May Also Like


