Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published October 2000R&D HorizonsThe latest radiology systems help recognize disease indicators during their early stages—when treatment may be most beneficial.William Leventon
October 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published October 2000
R&D Horizons
The latest radiology systems help recognize disease indicators during their early stages—when treatment may be most beneficial.
William Leventon
Life and death often depend on the smallest things. Tiny lesions, for example, can become deadly cancers if not detected and treated in time. The challenge for doctors is to locate and recognize small-scale trouble spots before they become large-scale problems.
Several new imaging technologies are intended to help doctors do just that. These technologies, used to look inside the body, scan for the smallest and earliest indications of disease. The techniques locate pathoformic structures that can be measured in millimeters—often microns.
One established technique of this kind is mammography, which is used for early detection of small, treatable breast cancers. But mammography isn't infallible. According to one estimate, mammography misses 10 to 15% of all malignancies. In cases where the test results are inconclusive, patients must usually undergo biopsy procedures. Although more than a million biopsies are performed each year, only about 20% of them detect the presence of cancer. This means that the vast majority of biopsies would not need to be performed if imaging methods could be made more effective.
AN AID TO MAMMOGRAPHY
Various techniques are used as adjuncts to mammography to enhance the performance of such systems. A new mammography aid known as transspectral impedance scanning, or T-scan, measures the movement of electricity through tissue. Because cancers have impedance values that are much lower than those of noncancerous tissue, they can be identified by the manner in which they influence the electrical current path.
T-scan technology can be used to locate tumors as small as 1 mm, according to Hal Kirshner, president of TransScan Medical Inc. (Ramsey, NJ), a company that manufactures a device based on the technology. Known as the T-Scan 2000, the device "can reduce the number of biopsies and also increase the number of cancers detected," Kirshner says.
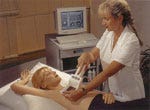
Figure 1. A handheld probe uses a transimpedance technique to measure a low-voltage current passing through the patient's body.
To perform the T-scan test, a patient holds an electrode, which emits a low- voltage (1- to 2.5-V) current that passes through the body (Figure 1). The electrical current is measured on the surface of the breast with a handheld probe that uses a transimpendance measurement technique. Detectors located on the probe pick up the electrical impedance, which is determined by measuring capacitance and conductance. The capacitance and conductance of malignant tissue have been found to be 50 times greater than that of either normal tissue or benign lesions.
Measurements are sent to a computer via an analog-to-digital convertor, and special algorithms are used to convert the data into capacitance and conductance images that appear in 256-level gray-scale format. The two-dimensional images are displayed in real time. Normal tissue and benign abnormalities appear gray in the image. Cancers, however, show up as bright white spots. The brighter the spot, the greater the likelihood that a malignant growth exists in that location (Figure 2).
Unlike conventional mammography, T-scan does not detect structural abnormalities like microcalcifications, which are often an indication of cancer. "We're not looking at the shape of the area or distortions in the shape. We're looking at the electrical properties," explains Peter Rotolo, TransScan's director of service operations. "If the electrical properties are a certain way, it could mean that there's a cancer in the area where mammography saw a microcalcification."
When used in conjunction with mammography, T-scan can significantly improve the accuracy of breast examinations, according to TransScan. Studies cited by the company show an increase of approximately 16% in sensitivity (the percentage of malignant lesions identified as "positive") and about 20% in specificity (the percentage of benign lesions identified as "negative") when a T-scan examination follows mammography. In addition, the T-Scan 2000 yields an accurate result 95% of the time when a lesion is determined to be benign, TransScan indicates.
T-scan has been found to offer advantages in several areas when compared with traditional mammography. Although mammography has limitations when used to image dense breast tissue, "T-scan measures capacitance and conductance in dense tissue just as it does in fatty tissue," Kirshner says. He notes that T-scan images require less interpretation than those produced by mammography. To make data evaluation less subjective, the system calculates conductance and capacitance and provides a readout of the figures.
T-scan is also noninvasive and requires no radiation or breast compression. The technique uses only low voltage levels, and the five-minute examination causes no discomfort. Most patients feel nothing at all, TransScan claims.
Kirshner admits, however, that system performance could be further improved. "We want consistently good sensitivity and specificity in detecting cancers and minimizing false positives," he says. "The system is accurate but not as consistent as we'd like it to be."
In the United States, the system has been cleared by FDA for use as an adjunct to mammography. Increasing the device's sensitivity and specificity, however, could support development of broader applications for the system. "We feel that the unit can be used as a breast screener," Kirshner says. "This is probably the only technology out there that has the potential to replace x-ray as a screening unit."
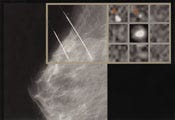
Figure 2. Cancerous tissue shows as bright spots in the T-scan's gray-scale images.
To reach that goal, TransScan is upgrading its software to use neural network technologies and proprietary algorithms based on data gleaned from thousands of T-scan examinations. "We're creating a way for the computer to get correct answers more often," Kirshner explains.
Aside from breast-cancer detection, T-scan eventually could have other uses. The firm is working on devices that will check for cancer of the skin and lymph nodes. The company may also put an impedance-measuring detector on the tip of a probe that could be used within the body.
LIGHT FOR LOOKING INSIDE
Another imaging technology in early development is based on using light from the terahertz band, located between the infrared and microwave regions of the electromagnetic spectrum (Figure 3). Because many materials are partially transparent to terahertz waves, terahertz-band emissions can penetrate objects to reveal information about them.
Until recently, it has been difficult and expensive to use radiation in the terahertz band. Innovative laser and semiconductor technologies, however, have opened this part of the spectrum to applications now being explored at Toshiba Research Europe Ltd. (Cambridge, UK). The Toshiba researchers believe that their terahertz pulse-imaging (TPI) system will yield diagnostic information that cannot be delivered by x-ray, MRI, or ultrasound systems.
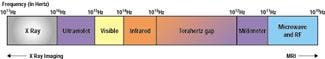
Figure 3. Light in the terahertz band, located between the infrared and microwave ranges of the electromagnetic spectrum, can penetrate objects to provide diagnostic information unavailable with MRI, ultrasound, or conventional x-ray systems.
To create terahertz light, the scientists use a pulsed laser that emits red light. The light is divided in two by a beam splitter; one beam is directed to a semiconductor, where red light waves of slightly different frequencies combine to create a beat frequency in the terahertz range. This terahertz beam reflects off of or passes through the sample to be imaged. To direct this light to the detector, the system uses the other half of the original beam divided by the splitter. The original red light beam mixes with the terahertz light from the sample in a second semiconductor. This semiconductor encodes the information carried by the terahertz beam into the visible beam, explains Don Arnone, Toshiba senior research scientist and terahertz group leader.
This data-carrying visible light can then be detected by a CCD camera or a photodiode. Both the detection equipment and the laser are off-the-shelf components. "We're using technology that's already available, and we're being slightly clever with semiconductors," Arnone says of the system.
The system can be used in two ways, the company suggests. When operated as an imaging device, the system shows three-dimensional shapes by measuring how the terahertz waves are affected by the structure of the sample. In this "time of flight" mode, the system measures the time required for the light waves to pass through the sample at each pixel in its image. By scanning the terahertz beam across the sample, a complete 3-D picture of the sample's internal structure can be created. This image can be displayed and manipulated on a computer screen.
TPI can also be used to identify the material inside a sample. This is accomplished by exposing the sample to a range of frequencies and examining how they are absorbed. The resulting absorption spectrum allows the material to be identified. According to Toshiba, conventional imaging techniques cannot match TPI's ability to provide a spectral fingerprint of an object at each pixel of its image.
Another advantage of TPI is that it is considered safer than conventional x-rays. Terahertz light is nonionizing, which means it does not change the chemical nature of the material it's probing. And power levels used for tissue imaging are usually lower than those of the background radiation that people are exposed to in everyday life.
EXPLORING THE POTENTIAL OF TPI
Currently, the Toshiba scientists are completing a prototype TPI system for detecting basal-cell carcinoma. TPI is considered to be better suited for this procedure than x-rays and ultrasound because it more readily detects water and blood content, Arnone explains. He adds that TPI is superior to MRI because it can detect structures as thin as 500 mm, while MRI cannot detect anything thinner than 1 mm.
Dental imaging is considered to be another promising area for TPI. The technique appears to be able to detect changes in water and chemical content associated with tooth decay. According to Arnone, TPI can detect tooth decay at an earlier stage than x-rays—often allowing decay to be treated without drilling.
In one mode, TPI can measure the thickness of tooth enamel; in another, it shows the internal tissue, or dentin, indicating the condition of the tooth cavity. The system can also create a 3-D image of the tooth, which can be rotated on a computer screen. In the future, this capability could help dentists examine a tooth from the optimal angle.
Arnone thinks it will be another one to two years before a TPI system is commercially available. Until then, the design team will be attempting to minimize the system's image-acquisition time. In the last year and a half, the team has reduced acquisition time from 10 hours to 7 minutes. This was accomplished by adjusting the semiconductor to increase the yield of terahertz power per unit of visible light power emitted by the laser.
Additional improvements are expected to be achieved by changing the way the system acquires an image. In its present configuration, the system requires an entire terahertz wave to create each pixel in an image. "But we don't need the full wave form, only one or two points on it," Arnone says. "By identifying the points we need and focusing on them, we'll be able to dramatically reduce the acquisition time."
The Toshiba team will also attempt to take advantage of techniques that other imaging modalities rely on. Because research is still at an early stage, the team has yet to incorporate time-saving algorithms and signal-processing techniques used by traditional x-ray, MRI, and ultrasound systems. "We're fairly confident that we can make the seven-minute-to-real-time jump in the next one to two years," Arnone says.
A more difficult challenge for Arnone and his colleagues is the presence of water, which absorbs waves in the terahertz range as they pass through tissue. Water absorption causes a particularly sharp falloff in high-frequency terahertz signals. The Toshiba researchers are attempting to use lower-frequency terahertz light to overcome this limitation. "As you go down in frequency, tissue penetration depth goes up," says Arnone, "and the absorption coefficient of water goes down." Unfortunately, image resolution decreases as well. "At 1 THz, you have a spatial resolution of 200 to 300 mm," Arnone adds. "If you go to 100 GHz, your spatial resolution is around 500 to 600 mm, maybe worse." The team hopes to identify an optimum frequency that maximizes penetration depth while maintaining adequate resolution.
Despite the limitations of TPI, Arnone believes that the technique may someday be able to handle breast screening. This is because terahertz waves can move relatively well through fatty tissue, penetrating 2 to 3 cm. A terahertz imaging system could conceivably reach farther into the body, however, by riding piggyback on an endoscopic probe. "We haven't developed these yet, but the technology for doing it is already available," says Arnone. "That's ultimately what we'd like to do."
SUPER-SMALL STRUCTURES REVEALED
TPI isn't the only light-based, limited-penetration imaging technology on the horizon. Optical coherence tomography (OCT) is also being explored as a promising new imaging modality. OCT measures the intensity of infrared light reflected from tissue depths of a few millimeters or less. Although the method cannot penetrate very far below the surface of tissue, OCT can produce images of unprecedented resolution, according to Paul Magnin, president of LightLab Imaging LLC (Westford, MA). LightLab holds the fundamental patents for OCT technology, which were acquired from the Massachusetts Institute of Technology (Cambridge, MA).
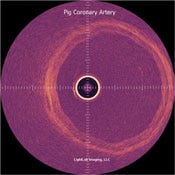
Figure 4. The image on the left of a porcine coronary artery shows the three layers of arterial wall–intima, media, and adventitia. The image on the right is a close-up view of the same artery, showing the three layers in greater detail.
OCT is similar to traditional ultrasound imaging, in which sound waves bounce off tissue to produce images. Instead of sound waves, OCT is used to direct light waves at tissue and measures how long it takes the light to return. This is performed with a system based on Michelson interferometer principles.
The system uses two light beams. One beam focuses on the sample, while the other provides a reference. Reflected signals interfere with the reference signal only when they come from a distance identical to the length of the reference arm, which is determined by the position of a mirror. By moving the reference mirror, the operator can get data from different sample depths. Signal-processing algorithms and hardware translate the data into 2-D or 3-D images that are produced in real time or close to it (Figures 4–6).
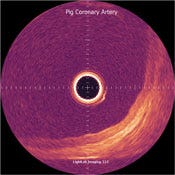
Figure 5. This image of the dissected human coronary artery shows where the intimal wall is separated from the medial wall.
LightLab indicates that current OCT systems have resolutions of 4 to 20 mm, compared with 110 mm for high-frequency ultrasound. The OCT technique is capable of providing images only of very small samples—about the size of a biopsy sample. Yet it detects such minute features that even images of small samples can generate significant amounts of data.
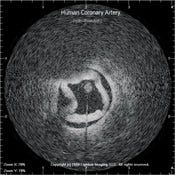
Figure 6. A histology image of the esophagus of a patient with Barrett's esophagus compared with an image generated using OCT technology.
Like TPI, OCT cannot look very deeply into objects. The photons of the system's light beam are scattered after traveling just a few millimeters through tissue. To overcome this limitation, LightLab is making transducers, catheters, and probes that will help OCT penetrate tissue more deeply and preserve the light signals traveling into and out of the body.
Magnin believes that OCT will prove useful in situations in which it is difficult to perform conventional biopsies. Coronary arteries, for example, "would be a great place for a nonexcisional biopsy," he explains. "You could look at a high-resolution image that tells you what's going on in that tissue, but without pulling a chunk out."
Someday, OCT could also offer a less-traumatic alternative to breast biopsies. "It's not easy to take a biopsy in the middle of the breast," Magnin notes. "Typically you have to cut in and dig a big hole to the lesion site." Using OCT, however, "you may be able to go in through a needle and get the diagnostic information you need without taking the biopsy tissue out."
Because the OCT system images through air, it does not have to touch the patient. According to Magnin, this differentiates it from ultrasound, which has to be in contact with tissue. Ultrasound also cannot be used in conjunction with surgical microscopes because of the air gap between the tissue and the end of the microscope, Magnin notes.
Although OCT received FDA clearance for use in microsurgery, the system's image quality is far from where Magnin wants it to be. "In no way are we ready to go yet," he explains, adding that a commercial OCT system is still about a year away. Once it's available, Magnin hopes that OCT will carve out its own niche in the imaging market: "OCT is a microscopic imaging technique, so it has a unique place in medicine."
CONCLUSIONS
Although T-scan, TPI, and OCT appear unlikely to overtake any of today's conventional imaging modalities, they could someday prove to be important additions to niche areas in medical diagnostic technology. Just a few years from now, if the developers are correct, these techniques will be used increasingly to assist physicians in the early detection of medical conditions ranging from tooth decay to cancer. By helping doctors see into the realm of the very small, this trio of imaging techniques is expected to play a key role in expanding and strengthening the armamentarium available to today's healthcare providers.
William Leventon is a freelance writer living in Somers Point, NJ.
Back to the MDDI October table of contents | Back to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like


