The hook-looking objects that appear on the nanoparticles’ surface are protein fragments that adhere to damaged vasculature.
March 1, 2010
Nanoparticles under development at MIT and Harvard Medical School could be used alongside stents, or in places that stents have difficulty accessing. This includes such hard-to-reach areas as near a fork in the artery, says Juliana M. Chan, a PhD student at MIT, who is working on the project.
Chan says that the nanoparticles may have applications to “atherosclerotic disease where revascularization procedures such as percutaneous angioplasty are performed to mechanically widen a narrowed or obstructed artery.” They may also be used to treat vascular injuries caused by cancers and inflammatory diseases.
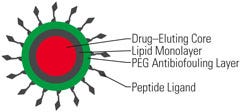
The research team calls the nanoparticles “nanoburrs” because they are coated with protein fragments (also known as peptide ligands or hooks) that help them stick to injured arteries. The nanoburrs are approximately 60 nm in diameter and have three layers (see Figure 1), Chan says. The inner core consists of a complex of paclitaxel, a chemotherapy drug, and a polylactic acid (PLA) polymer chain. The middle layer contains soybean lechithin, and the outer layer is made up of polyethylene glycol, “which protects the particle as it travels through the bloodstream and lets the particle circulate longer in the blood.”
The researchers designed the nanoburrs to target the basement membrane layer of damaged vasculature—a structure that is only exposed when the arterial walls are injured. To do so, the team covalently conjugated seven amino acid–targeting peptides on the nanoburr surfaces. According to Chan, “These peptides were discovered from a phage display screen against collagen IV to find affinity ligands to target our nanoparticles to injured vasculature.”
|
Figure 1. This diagram shows the composition of the nanoburr. PEG is polyethylene glycol. |
Once the nanoparticles find the basement membrane layer, they can deliver drugs like paclitaxel, which helps prevent the formation of scar tissue that can clog arteries, to treat the damaged vasculature. The researchers have some ability to control the timing of the drug release. Paclitaxel is linked to the PLA chain in the nanoburr’s inner core like a ball and chain, Chan explains. The paclitaxel is released when it detaches from the PLA chain, which happens gradually through a process known as ester hydrolysis. “The longer the chain, the longer it takes to release the drug, so if we increase the length of the chain, we can increase the time to release the drug,” she said.
In addition to delivering drugs for a long period of time, the nanoparticles can be injected intravenously at a location away from the injury. This type of treatment would eliminate the need for patients to undergo surgically invasive injections, Chan said.
|
Nanoburrs, which are shown here in electron micrograph pictures, are approximately 60 nm in diameter. |
The research team is currently working on efficacy studies in animals with injured vasculature. According to an MIT press release, a test showed that nanoburrs injected near the tails of rats were able to reach the intended target of walls of injured carotid arteries, but not normal carotid arteries. “The nanoparticles bound to the damaged walls at twice the rate of nontargeted particles,” the release said. The scientists are also considering the nanoburrs for other disease indications such as prostate cancer.
Chan is collaborating on this project with associate professor Omid Farokhzad, MD, of Brigham and Women’s Hospital and Harvard Medical School, and institute professor Robert Langer of MIT. Previously, the two researchers developed nanoparticles that target and destroy tumors. Their nanoburrs were featured in a January issue of the Proceedings of the National Academy of
Sciences.
About the Author(s)
You May Also Like