Originally Published MDDI June 2006 News Trends
June 1, 2006
News Trends
|
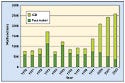
Figure 1. After falling off in the mid-1990s, the number of ICD malfunctions increased dramatically from 2000 to 2002. Source: CDRH Web site. |
CDRH and other organizations took significant steps in April toward overhauling how implantable heart devices are monitored and evaluated for safety after they are in use.
Major changes were expected after last year's recalls involving Guidant's and Medtronic's malfunctioning implantable cardioverter-defibrillators (ICDs). In Guidant's case, it was accused of being too slow to alert doctors and patients of the problem.
First, CDRH announced that it would expand its advisory panel on heart devices to include outside experts who will advise the center on postmarket safety issues. Their duties are expected to include helping CDRH interpret safety data and advising it on recalls.
Then in May the Heart Rhythm Society, which represents heart surgeons, published a report calling for sweeping changes to heart device safety monitoring. The proposed reforms include
• Having outside experts help determine when to issue safety alerts.
• Collecting more data about device performance.
• Standardizing the way in which doctors and patients are made aware of problems.
“Manufacturers are in the best position to evaluate their own devices,” the report stated. “However, a concern with this system is that the evaluation of the devices and the recommendations for actions by those within the company involves an inherent conflict of interest that could affect the outcome.”
What to do once authorities and caregivers are aware of a problem remains a sticky subject, though. A paper in the Journal of the American Medical Association (JAMA) raised questions about whether it is worth removing recalled implanted devices from patients. The study, conducted in Canada, found a 5.8% complication rate in procedures to remove recalled ICDs. A separate report in the same JAMA issue found that ICD malfunctions increased dramatically from 1998 to 2002, but declined from 2002 to 2004 (see Figure 1). Data for 2005, when the Guidant and Medtronic recalls were issued, were not available.
In a related matter, CDRH consolidated its postmarket surveillance guidances into a single document. It can be viewed at www.fda.gov/cdrh/osb/guidance/316.html.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like