January 6, 2014
The shrinking size of technology is fueling an array of advances that could transform the field.
Maybe call it the Gulliver's Travels effect, because cardiovascular technology is getting tinier. Innovators are working on everything from nanosensors that could detect heart attacks before they happen, to pill-sized leadless pacemakers.
While Lilliputian in scale, these advances are anything but laughable. Cadiovascular disease remains a grave problem, responsible for one in three deaths throughout the world.
Technological advances in cardiology over the past half century have improved and saved the lives of millions. But many treatments such as cardiac stents and cardiac rhythm management devices remain extremely expensive while leading to modest improvement in health for many patients. For instance, though stents can prove life-saving for heart attack patients, their efficacy in elective procedures is debatable in many cases. In September, Bloomberg ran an investigative article reporting that half of elective stents were unnecessary and had been tied to at least 773 U.S. deaths in 2012. As a result of these trends, sales of everything from stents to cardiac rhythm management devices have slowed while pricing pressures are cutting into the margins of the companies that make such products.
Even something as groundbreaking as transcatheter aortic valve replacement has concerns when it comes to costs, says Eric Topol, MD, a cardiologist who is the chief academic officer at San Diego-based Scripps Health. "I wish it was a catheter-based strategy that wasn't costing the same as a major open-heart operation. If that were the case, it would be even more attractive," Topol says.
Even catheter procedures themselves have challenges that are still being worked out, says Karen May-Newman, PhD, director of the bioengineering program at San Diego State University. "The cardiac surgeons are extremely deft and precise with their stitching. They know exactly the orientation. They can feel with their hands what the forces are and adjust the tension in their sutures," she explains. "You don't really have the same type of tactile feedback when you're placing something with a catheter. You also don't have, at least not yet, the ability to stitch precisely like you do when you actually have your fingers in there."
Topol think the miniaturization breakthroughs are happening at this time because all of the computer industry's advances with microprocessing chips and mobile device technology are increasingly spilling over into the medical device field.
There is also the issue of cost, May-Newman says. U.S. health reform and efforts in other countries have big payers such as Medicare requiring health providers to trim expenses. The result is a push for technology that is smarter and less invasive.
"Anything that lowers the cost of surgery, lowers the cost of hospitalization, is driving as many of these innovations as miniaturization. The two kind of go hand in hand anyway, because the smaller the cut you can make in somebody, the faster they should heal, the lower the complication rate," May-Newman says. (May-Newman is scheduled to chair a Learning Lab on cardiovascular devices on Wednesday, February 12, 2014, at MD&M West in Anaheim, CA.)
A Nanotech Heart Attack Detector
When it comes to saving both lives and money, nanosensors that detect heart attacks before they happen sound like a good deal. That is exactly what Topol at Scripps has been working on with Axel Scherer, PhD, of Caltech. Their technology involves tiny blood stream nano sensor chips that might sense the precursor of a heart attack. A person with such a tiny chip might get a warning on their smartphone or other wireless device that they should immediately see their cardiologist.
|
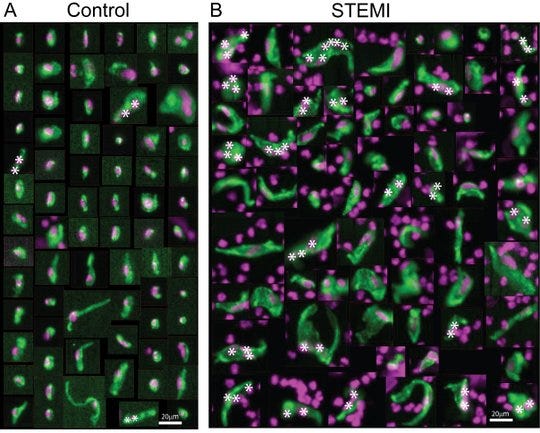
The appearance of circulating endothelial cells in heart attack patients (right) varies significantly from those of normal patients (left). |
The latest versions of the chip measure 90 microns--much smaller than a grain of sand. A doctor or nurse might inject the nanosensor into a patient's arm, where it would flow down to the distal tip of the finger and embed itself, screening the blood. "What it involves is a tiny sensor at the nano level that can be injected in the blood, that basically puts the blood under continuous surveillance," Topol says.
The basic premise is to use nanosensors to enable the same kind of realtime tracking of vital metrics that people take for granted when it comes to cars or computers. People get a warning when their car engines are burning up. Why not their own hearts?
"One of the things we're especially interested in seeing is endothelial cells that are sloughed off an artery wall that shouldn't be there in a healthy individual," Topol says. Endothelial cells are sloughed off in a precursory period preceding a heart attack.
"They're very unique and have a specific genomic signal. If we set up a sensor to pick up that signal, we can pick up an event potentially before it happens," Topol says. Because the technology can pick up endothelial cells from any source, it could be used to detect problems based in either the coronary artery or the cerebral arteries, making it potentially effective in detecting heart attacks as well as strokes.
In the case that a high level of endothelial cells are detected, the sensor could signal an alarm to the patient. "It may sound a little bit like the Amazon drone delivering your packages. But looks like it's technically doable," Topol says. "We've been working on this for a few years, and made considerable progress on the genomic signal and with our collaborators at Caltech on the sensor."
The sensors are now being tested in animal studies. Human trials should follow thereafter, Topol says. "The first project is not to pick up endothelial cells. It's just to do an important analyte, namely glucose," he says. "Once we have that and we can see for how long this sensor can last in terms of determining continuous glucose, then we're ready to start going after a genomic signal."
The combination of a nanosensor and coupled smartphone could be used be used to track autoimmune disease and cancer. It could also be used to screen for rejection in patients with organ transplants. In this application, the nanosensor could be calibrated to detect the donor organ DNA in the blood, which would begin showing up in the blood as an early sign of rejection.
The researchers have created two different sensor types: an electrochemical version and an optical sensing variety. "The good part here is we have a lot of diversification of ways to go forward," Topol says. "The issues are predominantly the accuracy of the signal detection and the durability of the signal detection. For a sensor in the blood, if it only lasts for a week or a month, that might not be as acceptable as it lasting for several months or a year at a time."
One of the challenges when developing the sensors is to make sure the devices are small enough to move through the blood stream to a capillary but large enough that they stay in the capillary and never makes it back to the heart. This would prevent the body from embolizing it. "There are lots of ways to deal with that. You can make sure that it sees blood, that it doesn't occlude blood flow. Every aspect of this, we have many different possible ways to solve this," Topol says.
Besides the immediate advantage of early warning signals for people, such sensors could provide important insights in the longterm.
Measuring cardiac data and even blood pressure continuously could revolutionize cardiologists' understanding of their patients. "I think that when you start to measure blood pressure in an individual seamlessly throughout the night, throughout their life, you're going to find a lot going on there we didn't know about," Topol says. "Just in this country, we have 70 million people with hypertension, but we don't even know if any of those people have controlled hypertension. And what about the other hundreds of millions of people? What's going on with them?"
"We're going to learn about what's normal, what isn't normal. Everything is kind of restarted when you have real-time continuous data that's seamlessly collected," Topol says.
Pill-Sized Pacemakers
Going smaller is also revolutionizing one of the most iconic cardiovascular devices--the pacemaker. Miniature electronics and catheter procedures appear to have reached a point together where the old method of running wires to the heart could become medical history.
And the devices are inserted inside the heart in a minimally invasive procedure, using a catheter.
Medtronic, Nanostim (recently acquired by St. Jude Medical), and Boston Scientific are all racing to introduce pill-sized pacemakers that could be implanted inside the heart in minimally invasive catheter procedures. Such devices would forgo the need for leads, which have long been the Achilles' heel of pacemaker devices.
For example, Medtronic has introduced several generations of pacemakers that have gradually become smaller and more efficient since it introduced the first external, battery-powered pacemaker in 1958. The company is now moving forward with studies on its own leadless pacemaker, the large vitamin-sized Micra transcatheter pacing system (TPS) that is only one-tenth the size of a standard pacemaker. The device is the product of more than seven years of internal R&D at Medtronic.
"When we first started, we didn't even know how to do half of the stuff. We just put the goal out there," says Mark Phelps, senior program director, diagnostics and monitoring at Medtronic. "Of course, everyone says you're crazy until you figure it all out, and then they say you're late," he jokes.
|
Hailing the Micra (shown on the right) as the world's smallest pacemaker, Medtronic developed the device in-house. |
The medical device giant recently announced that the device had been implanted in a human for the first time, with a patient in Linz, Austria receiving the device. Medtronic's latest study of the device involves a single-arm, multi-center global clinical trial that will enroll up to 780 patients at approximately 50 centers, with initial results from the first 60 patients expected in the second half of 2014.
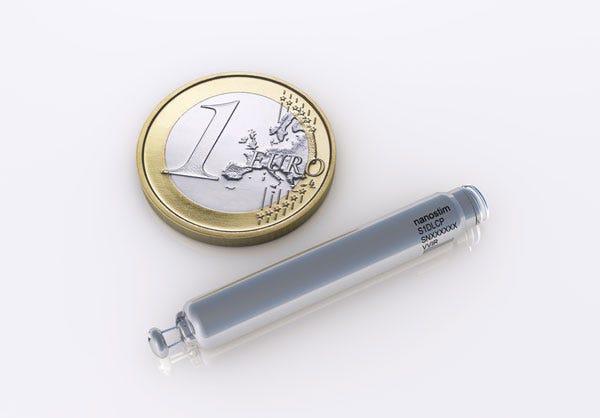
The early December news about the Micra implant came about two months after St. Jude Medical announced its $123.5 million acquisition of Nanostim, a Milpitas, CA-based company that has a AAA battery-sized device that won European Union approval.
Both the Medtronic and Nanostim devices forgo running leads to the heart, avoiding problems such as infection. The devices both have long battery life--10 years for the Micra and 13 years for Nanostim.
Medtronic took a more unusual approach to developing the Micra because it was a systematic, in-house effort. St. Jude meanwhile acquired the technology of an innovative young company, Nanostim (run by Drew Hoffmann, a former St. Jude executive), that had innovative technology.
In the case of the Medtronic Micra, designers concentrated on the systems linking the Micra in order to miniaturize it. "everything is connected to everything else," Phelps says. Instead of developing each component technology on its own path, the Medtronic team sought "deep miniaturization" as it worked to pull together the electronics, battery technology, packaging, electrodes, and case, and build them together as a system.
One example of how the miniaturization works involved the Micra battery. Instead of simply putting the battery inside the titanium case "can" as with older models, the battery became the titanium case in order to save space. "Now that the battery is the outside of the can, it also had to be the electrode," Phelps says.
Nanostim's top executive Drew Hoffmann meanwhile points to a key design breakthrough that enabled the company's pacemaker to go tiny: proprietary communications technology that allows the device to communicate patient information or receive programming instructions at much lower power levels than older pacemakers, enabling fewer components and more compact components.
"Our communication is conducted communication. It's kind of the way you measure an EKG. You measure the very strong signals emanating from a patient's heart to the surface of their body, with electrodes on the outside. ... I think our proprietary communication scheme and also the growing acceptance of catheters to do procedures in the heart involving devices like valves has opened the door to our approach," says Hoffmann, senior vice president and general manager of Nanostim at St. Jude.
Medtronic followed a similar approach: "We had to get ultralow-power electronics. That had to go all of the way back to semiconductor processes and tuning those," Phelps says. The devices run much slower than typical computers and smartphones, and thus save power. "We benefit from the slow signals that the human body puts out. But we do have to fine-tune the power. We measure every nanoamp in the system to make sure we don't waste any power at all."
The Micra--and a miniaturized implantable cardiac monitor that Medtronic developed over the same time frame--were designed to cut costs at several levels. For one thing, the technology was designed to be much easier for clinicians to deploy. And the devices' small size can potentially lead to faster healing for patients. "The leadless pacemaker goes in almost as a stent through the right ventricle into the atrium and gets attached. That's the whole procedure," Phelps says. "That would ordinarily be a pretty extensive operation: Make a pocket, put in the lead, sew up the pocket, and so on," he explains. "If you can go from an operating room to a procedure room, that's a pretty big difference in terms of cost."
|
One tenth the size of conventional pacemakers, the leadless Nanostim device won the CE Mark in late 2013. Its lifespan is comparable to that of standard pacemakers. |
Nanostim put a lot of working into making the implanting procedure something that would be very familiar to cardiologists and electrophysiologists, Hoffman says. "It's a catheter-based procedure. And we made a catheter that is very easy to use, that's able to be inserted into the femoral vein and then navigated into the heart, into the right ventricle," Hoffmann says. "And we have a number of features that give clinician flexibility in terms of where they put it in the heart and the ability to verify that it's working before they release it from the catheter."
At the same time, it was important that the Nanostim pacemaker have all the functions available in a conventional pacemaker, including constant stimulation, response to increased activity levels, and data storage, according to Hoffmann. "Patients like their pacemakers because they feel so much better after they've gotten one, and they've told me, 'I love my pacemaker, and I don't want to give up any of the function it has.'"
The ease of implantation bodes well for the global acceptance of a tiny pacemaker such as the Micra because it should be more affordable to implant as well as more effective, Phelps says. "For a country that doesn't have as many resources [as developed nations], simplifying the procedure and the venue gives those patients access to technology they didn't have before."
Chris Newmarker is senior editor of MPMN and Qmed. Follow him on Twitter at @newmarker. Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like