India is rapidly growing in several areas. Its 1.1 billion population is anticipated to surpass China in 20 years and U.S. medical device companies are more interested than ever in investing in this region. However, the country's advances are met with tough healthcare challenges.
November 23, 2010
|
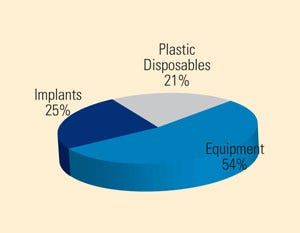
Breakdown of medical devices used in India, the majority of which are imported. Source: PricewaterhouseCoopers |
“India’s population has huge unmet healthcare needs—the country has a medical technology market about one-third the size of China’s, despite having roughly the same number of people,” says Kazuaki Kitabatake, president and CEO of Terumo America Holdings, in Ernst & Young’s Pulse of the Industry 2010 report. Compounding this challenge is the fact that only 10% of India’s population has health insurance, according to Sujay Shetty, director for pharma and life sciences, India, at PricewaterhouseCoopers (PWC). “Our industry’s challenge will be to continue to provide high-quality medical devices and diagnostics that more Indian patients can afford,” says Kitabatake of the import-dominated market.
Last year, the Indian government introduced the Central Devices Act to establish a body to create standards and regulate devices that are both made and used in the country, according to PWC. However, the body would govern high-risk devices, and low-end products would be self-regulated. The country’s 2010 Union Budget is supporting Indian manufacturers by increasing competitiveness among locally manufactured orthopedic implants and medical equipment.
In addition, India’s Drug Consultative Committee and the Drug Technical Advisory Board approved the Schedule M-III regulation, which defines medical devices as separate from pharmaceuticals. The Central Licensing Approval Authority serves as the medical device regulatory body and classifies devices. Devices that are imported into India are subject to the Schedule M-III regulations, but if they’ve been approved in the United States or European Union, they don’t have to go through additional conformity assessment.
|
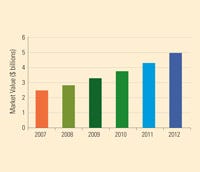
India's medical device industry is expected to hit $5 billion by 2012. Source: PricewaterhouseCoopers. |
India’s medical device market is only at the beginning of its rapid growth. As the new regulations aim to make a clearer distinction between devices and drugs, foreign and domestic parties are also encouraging the formation of an independent authority that regulates medical devices, according to PWC.
|
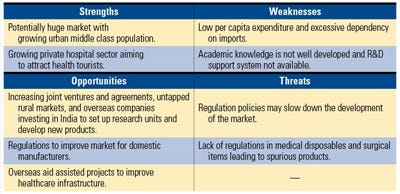
The Indian government is providing incentives for investment in the medical device market. Source: PricewaterhouseCoopers. |
About the Author(s)
You May Also Like