Technological innovation and corporate consolidation are altering the competitive landscapes of medtech’s hottest sectors.
January 1, 2007
BUSINESS PLANNING & TECHNOLOGY DEVELOPMENT
In 2007, market growth and technological advances in the medical device arena show no signs of slowing. Established sectors such as cardiovascular and orthopedics continue to see new growth engines on their horizons. Meanwhile, sectors such as neurotechnology—which is receiving unprecedented levels of attention from physicians and industry alike—are only beginning to realize the potential of emerging technologies. All the while, robust mergers and acquisitions activity continues to reshape the competitive landscape of the medical device industry as a whole.
As in years past, medtech executives will face new opportunities—as well as unprecedented challenges—in 2007. This article takes a look at what's on the horizon for some of medtech's key sectors this year and beyond.
Radical Change in IVDs
The global market for in vitro diagnostics (IVDs) is estimated to have exceeded $30 billion in 2005 and was on course to reach $32 billion in 2006 (see sidebar). Although overall growth remains in single digits at around 7%, the IVD market is entering a new era in which new technologies and new players will radically change the industry landscape and drive sales to more than $40 billion by 2010.
In 2006, the IVD market saw the entry of a new major player with the acquisition of Diagnostic Products Corp. (DPC; Los Angeles) and the diagnostics division of Bayer HealthCare (Leverkusen, Germany) by Siemens AG (Erlangen, Germany). The medical imaging giant paid ?4.2 billion (US$5.3 billion) for the Bayer business and $1.86 billion for DPC. The newly created Siemens Medical Solutions Diagnostics will be number two in the worldwide immunodiagnostics market. With leading positions in near-patient testing, laboratory automation, and hematology, the company will be competing for third place in the global IVD market, behind Roche and Abbott.
DPC's 2005 sales of $481.1 million, led by its Immulite immunoassay product line, placed it just outside the top-10 IVD companies, while Bayer ranked a joint third with revenues of $2.5 billion for its diagnostics and diabetes care units. Bayer retains its diabetes care division, which manufactures blood glucose- monitoring tests, but Siemens will get the diagnostics division, which generated sales of about $1.8 billion in 2005.
In 2005, Roche Diagnostics (Indianapolis) had an estimated 20% market share, followed by Abbott (Abbott Park, IL) with about 12% (see Table I).
Orthopedic Product Segment |
Reconstructive devices and joint replacements |
Spinal implants and instrumentation |
Fracture repair |
Orthobiologics |
Arthroscopy and soft tissue repair |
Other orthopedic products |
Total |
Table I. Global orthopedic revenues for 2005, by product segment. Figures may not add up due to rounding. Source: The Worldwide Orthopedic Market: 2005-2006, Knowledge Enterprises Inc. |
Although Siemens dominated the mergers and acquisitions news in the industry in 2006, another deal completed during the year saw Becton Dickinson (BD; Franklin Lakes, NJ) expand its presence in the cancer diagnostics market through its $350 million acquisition of TriPath Imaging (Burlington, NC). The deal followed the completion of BD's acquisition of GeneOhm Sciences (San Diego) in early 2006, which gave BD a foothold in the emerging field of healthcare-associated infections. BD also decided to quit the increasingly competitive blood glucose-monitoring market. Its monitors and test strips generated revenues of about $105 million in fiscal 2006.
In line with its focus on infectious diseases, cancer, and cardiovascular diseases, as well as industrial applications, bioMérieux Inc. (Marcy l'Etoile, France) sold its hemostasis range to Trinity Biotech (Dublin, Ireland) for an estimated $60 million.
The acquisition of Lumigen Inc. (Southfield, MI) by Beckman Coulter Inc. (Fullerton, CA) for $185 million gives Beckman assured access to Lumigen's chemiluminescent technology, which is used in Beckman's Access family of immunoassay systems.
Meanwhile, Tm Bioscience Corp. (Toronto) will have a new owner in 2007. In December 2006, Luminex Corp. (Austin, TX) announced that it had signed a definitive agreement to acquire the genetic testing company. Having developed the first multiplexed human disease genotyping test (for cystic fibrosis) to be cleared as an IVD for diagnostic use in the United States, Tm Bioscience is developing a number of tests for human genetics, infectious diseases, and pharmacogenomics. However, the company saw losses widen from $11.8 million in 2004 to $15.2 million in 2005.
Pharmaceutical giant Novartis (Basel, Switzerland) views its April 2006 acquisition of Chiron Corp. (Emeryville, CA) as a potential platform on which to develop molecular diagnostics. The deal highlights the convergence of the diagnostics and pharmaceutical industries in the development of personalized medicine. Pharmacogenetic tests will provide the key link in personalized medicine, identifying patients most likely to respond to a particular drug, develop resistance to treatment, or require dosage modifications.
|
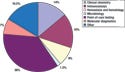
Figure 1. (click to enlarge) IVD market breakdown by segment in 2005. Source: Innovations in Diagnostics (London: Business Insights Ltd., 2006). |
Accounting for an estimated 7% of the global IVD market in 2005, molecular diagnostics is the fastest-growing segment of the industry (see Figure 1). From a base of $2.6 billion in 2005, it is projected to grow at a compound annual growth rate (CAGR) of 14% over the next five years. New technologies are moving molecular diagnostics beyond infectious-disease testing, tissue typing, blood screening, and genetic testing to molecular oncology, pharmacogenetics, and pharmacogenomics.
FDA and the European Medicines Agency are encouraging pharmaceutical manufacturers to incorporate pharmacogenomic tools into the drug development process. FDA has also produced a concept paper to provide a regulatory framework for tandem approval of companion drugs and diagnostic tests.
More genetic tests for personalized medicine are emerging from development. In May 2006, biotech company Jurilab (Kuopio, Finland) achieved CE marking for its DrugMEt pharmacogenetic test for clinical diagnostic use. The test will be made available by Jurilab's commercial partner, Nanogen (San Diego), for stratifying patient populations in clinical trials.
Point-of-care tests are also driving market growth. Accounting for a third of the global IVD market at almost $12 billion in 2005, tests used at the patient's bedside, in doctors' offices, and by the patient at home are forecast to grow at a CAGR of 7.8% over the next five years. Micro- and nanotechnologies promise to revolutionize point-of-care testing, provided the technology addresses market needs for simple-to-use devices, rapid results, better diagnostic data management and connectivity, robust quality controls, and affordability.
The entrance of new players such as Siemens and greater synergies between pharmaceutical manufacturers and diagnostic companies will blur the boundaries between in vivo diagnostics, IVDs, and pharmaceuticals. Technology innovations are increasing the pace of change, producing tools that enable earlier and more-accurate diagnosis of disease, improved clinical decisions, and better monitoring of treatment. Adoption of innovative technologies in the marketplace, however, will need to overcome hurdles in reimbursement and regulatory policies, which are not keeping pace with the development of innovation.—Jeanette Marchant, principal, JM Communications
Orthopedic Opportunities Still Robust
Despite the fact that the orthopedics sector continues to post year-over-year gains that would be the envy of most manufacturing industries—including some medical device sectors--many medtech analysts have focused on the declining rate of growth of the orthopedics market. But while annual growth rates for the orthopedics sector have decelerated somewhat from the near-20% levels of a few years ago, the sector is still setting a robust pace. Worldwide sales of orthopedic products in 2005 generated revenues of $25.9 billion, with every major segment reporting double-digit growth. Such growth was led by the spine segment with an 18.9% increase over 2004. Overall, 2005 sector revenues topped year-earlier performance by 12.7%.1 Market estimates placed 2006 global revenues for the sector around $28.2 billion.
The United States accounts for 70.2% of the global orthopedics market, followed by Europe (17.1%) and Asia-Pacific (12.8%). The Asia-Pacific market for orthopedics is dominated by Japan, which has a near-80% share in the region.2
Worldwide there are more than 200 orthopedic manufacturers, with about 80 firms posting annual revenues of $5 million or more.1 Accounting for about 75% of the global market, the top-10 companies rang up revenues of $19.5 billion during the 12-month period ending on September 30, 2006—a 9.2% increase over the year-earlier period.
DePuy Inc. (Warsaw, IN), a Johnson & Johnson company, led the industry during the period with a little more than $4 billion in revenues. In addition to DePuy, six other companies dominate the industry: Zimmer Holdings Inc. (Warsaw, IN); Stryker Corp. (Kalamazoo, MI); Synthes Inc. (Solothurn, Switzerland); Medtronic Inc. (Minneapolis); Biomet Inc. (Warsaw, IN); and Smith & Nephew plc (London). The remaining three companies on the top-10 list—DJO Inc. (Vista, CA), Encore Medical Corp. (Austin, TX), and Wright Medical Group Inc. (Arlington, TX)—generated combined revenues of $1.07 billion, or just 5.5% of the total revenue generated by the top 10 companies (see Table II).
Company |
DePuy Inc., a Johnson & Johnson company |
Zimmer Holdings Inc. |
Stryker Corp. |
Synthes Inc. |
Medtronic Inc.a |
Biomet Inc.b |
Smith & Nephew plc |
DJO Inc. |
Encore Medical Corp.. |
Wright Medical Group Inc. |
Total |
a12-month period ending October 27 |
Table II. Revenues of the top 10 public orthopedics firms for the 12-month period ending September 30, 2006, versus the previous 12-month period. Revenues are from sales of orthopedic products only. Source: company quarterly financial reports. |
Merger and acquisition activity in the orthopedics sector was down in 2006 compared with the previous year. Nevertheless, the sector's major players produced a big surprise at year's end. Shortly after Smith & Nephew acknowledged that the company had made a bid for Biomet in early November, offers from other suitors apparently upped the ante, causing Smith & Nephew to exit negotiations. A group of private equity investors—including Biomet founder and former CEO Dane Miller, who was forced out of the company in March 2005—emerged as the new buyers. Shortly after the deal was announced, industry analysts flagged Smith & Nephew as a possible takeover target.
Although the reconstructive-device and joint-replacement implant segment has experienced slowing growth over the past two years, it continues to lead the orthopedics sector with 2005 sales of $9.6 billion and a 37.1% share of overall revenues.
"More than 2 million joint- replacement procedures took place in 2005," says Shirley A. Engelhardt, president and founder of Knowledge Enterprises Inc. (Chagrin Falls, OH), publisher of the OrthoKnow and Bare Bones industry newsletters. "The world's six largest joint-replacement companies—Biomet, DePuy, Smith & Nephew, Stryker, Wright Medical, and Zimmer—control 89% of the global market. Yet there are more than 100 other companies that have built respectable joint-replacement franchises by focusing on particular geographic regions or specific types of implants."
Spinal implants and instrumentation accounted for 16.7% of the global market, while fracture repair took 11.6%, orthobiologics took 9.3%, arthroscopy and soft-tissue repair took 8.5%, and other orthopedic products took 16.7% (see Table III).
Orthopedic Product Segment |
Reconstructive devices and joint replacements |
Spinal implants and instrumentation |
Fracture repair |
Orthobiologics |
Arthropscopy and soft tissue repair |
Other orthopedic products |
Total |
Table III. Global orthopedic revenues for 2005, by product segment. Figures may not add up due to rounding. Source: The Worldwide Orthopedic Market: 2005–2006, Knowledge Enterprises Inc. |
Growth in the orthopedics sector is intrinsically linked to significantly increased life spans in populations around the world. Often a product of the aging process, osteoarthritis is a leading cause of joint deterioration and a key driver in the demand for knee, hip, and other joint replacements. While the joint-replacement market is still dominated largely by the age 60-and-over population, orthopedic surgeons continue to report a small but discernible increase in middle-aged and younger patients who are seeking relief from the pain and debilitation of joint disease. The rise of sports medicine, typically focused on younger populations, is also driving industry growth. Such growth is occurring on the surgical front, due to bone breaks and fractures associated with injuries, as well as on the nonsurgical front, which includes external bracing and immobilizing devices.
With back pain affecting an increasing number of patients around the world, the orthopedics industry continues to introduce an array of innovative devices and surgical instruments to both ease the suffering associated with the condition as well as return the patient to a greater range of functional mobility. Spinal fusion surgery is increasingly benefiting from less-invasive procedures that result in smaller incisions, less blood loss, shorter hospital stays, fewer postoperative complications, and faster rehabilitation and recovery times.
Artificial spinal disks also offer promise for back-pain patients. Although the Charité disk from DePuy Orthopedics has met with reimbursement resistance from Medicare, private insurers, and some orthopedic surgeons, new products are expected to overcome some of the problems associated with first-generation devices. The ProDisc from Synthes, which has both FDA approval and favorable reimbursement coverage, has been generally well received by industry analysts. Hoping to build a solid base of support, the company has established clinical centers around the country to train orthopedic surgeons on the proper technique and procedure for disk implantation.
The spine sector continues to be orthopedics' hottest segment. While it too has slowed from the torrid pace of a few years ago, when year-over-year growth was pushing 30%, "It's still the place to be," says John McCormick, medtech analyst and a managing director at Healthpoint Capital LLC (New York City), an investment banking firm specializing in orthopedics.
Consolidation is also going on in the spine segment, as larger players seek to tap into newly emerging technologies. At the end of 2006, Kyphon Inc. (Sunnyvale, CA) announced plans to acquire spinal device manufacturer St. Francis Medical Technologies Inc. (Alameda, CA) for approximately $525 million, plus future revenue-based contingent payments of up to $200 million. Later that month, the company signed two definitive agreements to acquire all of the spine-related product assets and associated intellectual property rights of privately held Disc-O-Tech Medical Technologies Ltd. (Herzeliya, Israel) and its U.S. subsidiary for approximately $240 million. With these additions, Kyphon is likely to break into the top-10 list of orthopedics sector players in 2007.
While reimbursement rates from government and private insurers continue to squeeze profits—particularly for large-joint replacements such as total knee and hip arthroscopies—cuts in reimbursement rates have not caused a cutback in procedures. "Check the data," McCormick says. "Assuming you have a clinically viable device embraced by the surgeon community, the correlation between reimbursement and gains or losses in orthopedic procedures just isn't there. Many industry watchers confuse volume with prices. Pricing pressures from hospitals are squeezing manufacturer profits and surgeon fees, but that's really a separate issue. The volume increases in the orthopedics space continue to be constant—and typically growing year after year. Due to favorable demographics that are not going away, growth is basically built in to the fundaments of the orthopedics market."
With knee and hip replacements no longer commanding their premium prices, the small-bone segment is showing increasing promise as a new growth area for joint-replacement technologies. According to John Viscogliosi of Viscogliosi Brothers LLC (New York City), an investment banking firm centered on specialty orthopedics, the small-bone segment is an underserved market that is generating significant surgeon and industry interest. "Since we launched Small Bone Innovations in early 2005, about 25 to 30 new companies have been formed that focus on this area, which was formerly referred to as extremities."
In addition, gainsharing continues to spur discussion in the orthopedics sector. Under some models, gainsharing arrangements provide financial incentives to doctors who agree to use preapproved medical devices, equipment, and supplies that have been standardized by hospitals to control costs through volume buying. The U.S. Department of Health and Human Services Office of Inspector General (Washington, DC) initially ruled such arrangements illegal, but it has recently approved a number of gainsharing demonstration projects on a carefully controlled basis. To date, the impact on the orthopedics sector has been minimal.
Direct-to-consumer (DTC) advertising is making steady inroads in orthopedics and is generally considered to be a growth driver, albeit on a relatively small scale at the present time. While patients reportedly see such campaigns as information resources, many surgeons are concerned that the ads present only the positive aspects of orthopedic procedures and risk compromising the doctor-patient relationship. While both FDA and the Federal Trade Commission continue to monitor the situation, DTC advertising looks as though it will play an increasing role in orthopedics marketing in the years ahead.
Despite their frequent preoccupation with the diminishing rate of growth in the orthopedics sector, most industry analysts remain bullish for both the near- and long-term future of the sector. By 2010, the sector is expected to produce $44.7 billion in global revenues, an increase of more than 166% over the seven-year period beginning in 2003. Growth in 2006 was estimated to be about 12%. Going forward, an uptick to a 15% annual growth rate is forecast through 2010.3
The orthopedics sector benefits from favorable demographics, including more than 70% of the world's population remaining as prospective new customers. And companies in the field have created a dynamic product pipeline that is turning out a steady stream of advanced design materials, precision surgical instruments, biologics, and innovative reconstructive devices. On the basis of such strengths, the orthopedics sector is poised to continue its enviable financial performance record for the foreseeable future.—Art Kerley, president, Fairfield Factor Inc.
Cardiovascular Opportunities and Obstacles
With a global valuation of about $27 billion, the cardiovascular market is currently growing at an annual rate of 16%.4 In the North American market, cardiovascular devices generated revenues of $17.88 billion in 2005. With an aging population that has a greater incidence of cardiovascular disease as a key driver, the North American cardiovascular market is expected to reach $40.46 billion by 2011.5 The American Heart Association (Dallas, TX) estimates that 64 million Americans have some form of cardiovascular disease.
The cardiovascular sector includes coronary stents, cardiac rhythm management (CRM) devices such as pacemakers and implantable cardioverter-defibrillators, prosthetic heart valves, vascular grafts, ventricular-assist devices, self-contained artificial hearts, angioplasty catheters and balloons, and other clinical support devices. Accounting for nearly two-thirds of the sector, coronary stents and cardiac rhythm devices by far dominate the category.
Stent Evolution. The coronary stent was introduced to the U.S. market in 1994. Although effective in restoring adequate blood flow through the diseased artery and relieving angina and other symptoms, first-generation stents were subsequently linked to restenosis, which is the buildup of scar tissue at the site of implant. Although stent geometries steadily improved, a major advance came in 2003 with the introduction of the first drug-eluting stent—Cypher, developed by Cordis Corp. (Miami Lakes, FL), a Johnson & Johnson company. A year later, Boston Scientific Corp. (Natick, MA) gained approval from FDA to introduce its Taxus drug-eluting stent.
In introducing the devices, manufacturers of drug-eluting stents claimed that the drug-device combination products reduced the occurrence of restenosis by slowly emitting a therapeutic drug. As the efficacy of drug-eluting stents was repeatedly demonstrated in clinical studies, cardiologists embraced the devices and the market took off. A number of medtech companies jumped into what rapidly emerged as a highly competitive and contested market characterized by an extensive series of patent infringement suits regarding stent designs. By 2005, drug-eluting devices had captured 85–90% of the coronary stent market. The worldwide drug-eluting stent market is currently valued at about $6 billion.4
The good times for drug-eluting coronary stents were seriously challenged last year following clinical presentations at the 2006 World Congress of Cardiology, in Barcelona, Spain. The findings, which suggested a significant risk of late-term thrombosis associated with the devices, were widely and prominently reported in industry, business, and popular media. As cardiologists and their patients grew concerned about the safety of the devices, usage reportedly declined and analysts expressed concern about the near- and long-term impact on the market.
However, clinical studies reported at the Transcatheter Cardiovascular Therapeutics (TCT) conference in October and a meeting of FDA's circulatory devices panel in December gave drug-eluting stents a new lease on life. Findings presented during TCT generally concluded that the devices showed no increased risk of thrombosis and resulting myocardial infarction or death when compared with bare-metal stents. Similarly, the FDA panel, while acknowledging an apparent increase in stent thrombosis, found that drug-eluting stents were generally safe when used as directed. The panel members expressed concern about reported extensive off-label use of the devices. They also called for increased postmarket studies and surveillance. But they did not recommend any significant changes in clinical trials or the premarket approval process for new drug-eluting coronary stents seeking entry to the U.S. market.
Other drug-eluting stents poised for FDA approval later this year or in early 2008 include Endeavor from Medtronic Inc. (Minneapolis); Xience, manufactured by Abbott (Abbott Park, IL); and CoStar from Conor MedSystems Inc. (Menlo Park, CA). Conor has recently agreed to be acquired by Cordis Corp. for $1.4 billion.
"The companies in this segment and the cardiologists who implant the devices both seem generally pleased with actions of the FDA panel," says Jan Wald, PhD, medtech analyst with A.G. Edwards Inc. (St. Louis). "Right now, they're as comfortable as they can be. The penetration rate for drug-eluting stents, which was as high as 88%, has reportedly fallen into the 70% range.
"But there are some innovative products in the pipeline, including the CoStar stent from Conor Medsystems, which has a unique drug-eluting technology, as well as a design that utilizes bioresorbable polymers that are absorbed by the body after the drug is released," Wald adds. "The stent has been well received by cardiologists, which explains why Cordis put Conor on its shopping list."
Wald says coronary stent development will continue to show innovation in design. Third-generation devices, such as Abbott's completely bioabsorbable drug-eluting stent, offer great promise for the sector going forward. The Abbott stent, made of polylactic acid, is designed to be completely absorbed by the body. In contrast, only the polymer coating on the CoStar is absorbed, leaving the metal scaffolding of the stent in the artery.
Wald also acknowledges that safety concerns regarding drug-eluting stents could provide an opening for cardiac surgeons to promote coronary artery bypass surgery, which has been in decline since the advance of interventional cardiology technologies. Meanwhile, new tools and procedures have also made open-heart surgery less invasive. The beating-heart procedure, also known as off-pump surgery, is not new, but it is gaining renewed attention. The procedure does not require placement on a heart-lung machine and is generally regarded as being less traumatic for the patient.
CRM Potential. On the cardiac rhythm management front, 2006 proved to be a stabilizing year. Following extensive recalls of Guidant's implantable cardioverter-defibrillators and cardiac pacemakers in 2005, a task force of manufacturers, regulators, cardiac care providers, and patient advocacy groups was organized by the Heart Rhythm Society (Washington, DC) in cooperation with FDA. The group developed comprehensive recommendations for the surveillance, analysis, and performance of CRM devices. Industry stakeholders uniformly embraced the resulting recommendations.
The U.S. cardiac rhythm management market produced revenues of $5.99 billion in 2005. The U.S. CRM market is forecast to reach $16.79 billion by 2012.6
CRM devices are thought to have significant potential for market growth outside the United States. In Europe, where adoption rates have lagged, markets for the devices are now poised for significant growth. Asian markets—China, in particular—are expected to see gains as well.
CRM devices are increasingly incorporating advanced pacing and programming technologies, which ensure adequate levels of electrical stimulation while minimizing unnecessary shocks. Less-invasive implanting and device removal procedures are expected to provide greater patient comfort and confidence, which will further grow the rate of adoption worldwide.
Leading CRM manufacturers include Medtronic, St. Jude Medical Inc. (St. Paul, MN), and Guidant, which is now a wholly owned business unit of Boston Scientific.
Valve Stability. In the heart valve segment, biological technologies continue to make inroads in the $1 billion market.7 Yet mechanical prosthetic devices still dominate the market with a 60% share—a trend that MedMarket Diligence principal Patrick Driscoll says is not likely to change despite the advances in biological valves.
"The durability of mechanical valves, which can be for the lifetime of even a younger patient, comes at the expense of hemolysis and the requisite use of anticoagulants," he says. Driscoll cites a number of advances on the mechanical front, but adds, "No innovation has had an appreciable impact on the coagulation caused by mechanical valves."
Percutaneous heart valve technology, which enables the interventional implantation of devices without the need for major surgery, is currently one of the most intensely researched product development areas in the cardiovascular sector. By opening the replacement valve market to patients deemed unsuitable for open-heart surgery, percutaneous and other novel valve technologies are expected to provide significant growth opportunities going forward.8
Leading players in the prosthetic heart valve market include Edwards Lifesciences Inc. (Irvine, CA), St. Jude Medical, Medtronic, Sorin Group (Milan, Italy), ATS Medical Inc. (Plymouth, MN), CryoLife Inc. (Kennesaw, GA), MedicalCV Inc. (Inver Grove Heights, MN), and Evalve Inc. (Menlo Park, CA).
Continued Assistance. Heart-assist devices represent one of the smallest segments of the cardiovascular sector. While a great deal of the activity in the segment is experimental, there are a number of commercially available ventricular-assist devices on the market, which is expected to reach $350 million in the United States by 2009.9 By extending or replacing the pumping function of a damaged heart, these devices can sustain patients' quality of life while they await the availability of suitable transplants.
New advances in cardiovascular therapies have also enabled heart-assist devices to take over for a potentially recovering heart. Since the number of patients with failing hearts far exceeds the supply of transplants, one of the goals of this segment is the development of a completely self-contained artificial heart. Such a device—the AbioCor, manufactured by Abiomed Inc. (Danvers, MA)—was approved by FDA in 2006 under a humanitarian device exemption for limited and highly controlled use. Abiomed also manufactures ventricular assist devices. Other companies producing heart-assist devices include Thoratec Corp. (Pleasanton, CA), World Heart Corp. (Oakland, CA), and MicroMed Technology Inc. (Houston).
Overall, the cardiovascular sector continues to evolve. New advances in materials, biocompatible coatings, and drug-delivery technologies are expected to spur the advance of more-innovative coronary stent designs. In addition, steadily improving imaging techniques will provide cardiologists with enhanced image acquisition and analysis for in-stent visualization and more-accurate diagnoses and recommended treatment. Increasing use of onboard electronic sensors will monitor the programming and safety performance of CRM devices while providing enhanced arrhythmia management.
With cardiovascular disease accounting for the greatest outlay of healthcare expenditures, the sector presents a dynamic opportunity for medtech manufacturers to develop diagnostic and therapeutic devices that are less invasive, enable shorter hospital stays, and speed patient recovery—all while reducing costs for patients, payers, and providers.—Art Kerley, president, Fairfield Factor Inc.
Significant Potential in Neurodevices
The neurodevice market is one of the fastest-growing sectors of the overall medical device industry. Global neurodevice sales in 2005 were approximately $3.4 billion, representing growth of about 21% over the prior year.10 Neurodevices include medical devices, electronic implants, surgical equipment, and software systems designed to treat brain and nervous system illnesses. Currently approved devices include deep-brain stimulators for Parkinson's disease, spinal cord stimulators for pain, and cochlear implants for deafness.
The neurodevice sector consists of four segments: neuroprosthetics, neurostimulation, neurosurgery, and neurofeedback (see Table IV).
Segment |
Neuroprosthetic |
Neuro- |
Neurosurgical |
Neurofeedback |
Table IV. Descriptions and global revenue estimates of neurodevice segments. Source: NeuroInsights. |
Neuroprosthetics are electromechanical devices that interface with the nervous system to compensate for a sensory deficiency or motor deficiency. Sensory deficiencies like deafness and blindness can result from damage to the sensory organ, the sensory nerve connecting the organ to the brain, or the sensory cortex in the brain. If the damage is in the first two areas, neurodevices can be used to directly stimulate the sensory nerve or even the sensory cortex. The neuroprosthetics market is currently dominated by cochlear implants, which had estimated worldwide sales of $450 million in 2005, representing 21% growth over 2004.10
Neurostimulation devices generated sales of $1.2 billion in 2005 with 16% growth.11 Neurostimulation devices are electrical and magnetic stimulation devices that include deep-brain stimulators for Parkinson's, spinal cord stimulators for pain, vagus nerve stimulators for epilepsy, and peripheral-nerve stimulators for urinary incontinence and obesity. Currently, most revenue in this sector comes from patients with chronic pain, movement disorders, and epilepsy. In the next three years, patients with severe depression, migraine headaches, and obsessive-compulsive disorders can expect neurostimulation devices to be approved by FDA. New neurostimulation treatments for patients with chronic anxiety, obesity, bulimia, and Alzheimer's disease are a bit further out on the horizon (see Table V).
Company |
Boston Scientific |
Boston Scientific |
EnteroMedics |
IntraPace |
Leptos |
Medtronic |
Medtronic |
Medtronic |
Medtronic |
Neuralieve |
Neuronetics |
NeuroPace |
Northstar Neuroscience |
Northstar Neuroscience |
Northstar Neuroscience |
Northstar Neuroscience |
St. Jude Medical |
St. Jude Medical |
Table V. Select neurostimulation device clinical trials underway at press time. Source: NeuroInsights. |
The market for neurosurgical medical devices totaled roughly $1.75 billion in 2005, and is projected to grow at a rate of 20% annually.10 This category includes minimally invasive surgical tools, surgical navigation devices, neurovascular interventions, and other medical supplies for neurological diseases—such as shunts for hydrocephalus and peripheral-nerve repair guides.
The convergence of software, imaging, and brain research is leading to new neurofeedback and software-based solutions for a wide range of cognitive, emotional, and sensory disorders. If companies in this space can demonstrate efficacy and connect with customer concerns about side effects from neuropharmaceuticals, they have the potential to become first-line treatments for disorders like attention deficit hyperactivity disorder (ADHD) and mild cognitive impairment. There are strong drivers for rapid growth of this sector, including the following.
Noninvasive, zero-side-effect treatment, particularly for children.
Based on high profit margins, fast return on investment, and lack of current regulatory involvement, venture capital investment is expected to be significant.
Participation and marketing by the $23 billion video game industry and learning games industry will accelerate market adoption.
The large computer-literate baby boomer population concerned about mild cognitive decline may turn to neurofeedback while neuropharmaceutical treatments remain in development.
Target revenue from direct-to-consumer distribution for home use and through learning centers or clinics.
The number of neurodevices registered with FDA across all segments rose more than 400% from 1998 to 2005, compared with only a 16% increase for overall medical device registrations.10 Advances in technology paired with increased market uptake are driving sustained market growth of neurodevices and penetration into new disease indications, including stroke, obesity, and depression. Critical industry challenges facing neurodevice companies include patient bias against implantation, reimbursement, and the need to educate clinicians. Growth drivers in the neurodevice sector include the following.
Technology. Microelectronics for smaller devices, wireless technology for reprogramming improvements in device operation, rechargeable long-life batteries, and micro- and nanoelectrodes that can connect to the nervous system are all driving improvements in the efficacy and tolerability of implantable neurodevices.
Regulatory Issues. Devices generally have fewer unexpected side effects than neuropharmaceuticals, leading to fewer late-stage failures and shorter clinical trials.
Start-up Financing. Neurodevice companies, which generally get products through FDA approval more quickly and with a smaller investment than neuropharmaceutical companies, are favored by certain venture capitalists.
Patient Adoption. Neurodevices are being approved for treatment-resistant patient populations that are willing to take additional measures beyond drug therapy to relieve their symptoms. Medical device manufacturers are dedicating large marketing budgets to educating and training clinicians and patients. Technical improvements to make devices smaller and more effective are also contributing to patient adoption.
Reimbursement. While the cost of a neurodevice can range from $10,000–$50,000 per implant, pharmaceutical treatments might cost $10–$50 per day. Traditionally, insurance companies have been hesitant to pay for these upfront costly procedures, but new studies are showing that current devices with low maintenance schedules can justify the cost by reducing long-term pharmaceutical and physical therapy costs.
Competitive Landscape. No single company participates in all four neurodevice segments. This stands in contrast to other medical device markets, which are generally dominated by a few major players. For example, Boston Scientific Corp. (Natick, MA) provides most products in interventional cardiology. The current fragmentation of the neurodevice market portends increased mergers and acquisitions activity (see sidebar).
Target Customers. In the United States, purchasing decisions for neurodevices remain with physicians. The key purchasing groups include neurosurgeons, neurologists, pain specialists, spine surgeons, and interventional neuroradiologists.
Overall, the neurodevice sector will continue to see strong growth as technology advances make it possible to treat an ever-larger number of neurological and psychiatric illnesses with greater effectiveness.—Zack Lynch, executive director, Neurotechnology Industry Organization (San Francisco).
Imaging Opportunities, Big and Small
In the United States, market demand for medical imaging equipment is expected to reach more than $16 billion by 2010, with a forecast CAGR of 6.8% between 2005 and 2010. By 2015, U.S. market demand for imaging equipment is expected to top $21 billion.
Computed tomography (CT) is expected to lead the sector's growth, with a CAGR of 10.3% between 2005 and 2010. By 2010, U.S. market demand in the CT segment will reach $3.3 billion (see Table VI).12
Medical Imaging Segment
Computed tomography
Magnetic resonance imaging
Medical x-ray
Nuclear medicine
Ultrasound
Fluoroscopy
Positron emission tomography
Other equipment
All equipment
Table VI. Actual and forecast U.S. market demand and compound annual growth rates for medical imaging equipment, 2000–2015, by segment. Source: The Freedonia Group Inc.
The annual meeting of the Radiological Society of North America (RSNA) in late November is the traditional launchpad for products in every imaging technology, including x-ray, computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and positron emission tomography (PET). Devices, applications, and news emerging from the event provide a solid gauge of the current state of the imaging sector, as well as its future directions. The 2006 RSNA conference had a total attendance of 61,294, down slightly from last year's attendance of 61,565. Exhibitors outnumbered professional registrations, with 28,044 vendors compared with 27,138 professional attendees.
For the second year running, molecular imaging was a major undercurrent at the show. Capable of providing images at both the cellular and molecular levels, molecular imaging technologies provide a functional view of the body compared with traditional modalities that reveal internal structures. Disruptive technologies with implications for radiology, oncology, and cardiology, molecular imaging modalities are used across the spectrum of care to provide diagnostic data and support drug therapies and surgical interventions. The numerous modalities used for molecular imaging—including nuclear, PET, and MRI—highlight the pervasive impact molecular imaging will have on physicians, providers, and vendors.
New developments in molecular imaging will increase the number of imaging procedures and expand the use of imaging modalities beyond traditional diagnostic applications. At the 2006 RSNA meeting, a number of molecular imaging research studies were presented. As is often the case with new fields, the presentations focused on technique development and applications. Most of the large vendors touted collaborative research. One promising technique presented was the magnetic resonance imaging of superparamagnetic iron oxide (SPIO) particles, which holds promise for improving in vivo cell localization and tracking as part of emerging stem cell therapies. Another topic covered in a number of research presentations was the use of molecular imaging for measuring the efficacy of specific cancer therapies. A number of studies presented findings on the use of proton (1H) and phosphorus-31 (31P) magnetic resonance spectroscopy (MRS) for in vivo detection of therapeutic responses to chemotherapy.
Developments in image management and information technology were also front and center at RSNA 2006. As the picture archiving and communications systems (PACS) market matures, vendors are moving to consolidate radiology information systems (RIS) and PACS into integrated systems (see sidebar). Additionally, in recent years there has been a lot of acquisition activity in the cardiovascular information systems market. Companies making acquisitions in this market—including McKesson, Agfa, Philips, and Emageon—report that their product roadmaps include integrated radiology-cardiology image management solutions that will become available in the mid term.
One issue simmering beneath the aforementioned product evolutions—an issue that many buyers have yet to fully appreciate—is data migration. As study volumes and average study sizes increase, hospitals and manufacturers are going to find data migration and scalability of current storage solutions of increasing importance. Over the next few years, buyers will be challenged to avoid hidden costs and product roadmap dead ends.
Another underlying theme at RSNA was the strategic targeting of unmet or poorly met niche markets by start-up companies. While behemoths like Siemens Medical Solutions (Malvern, PA), Agfa-Gevaert NV (Mortsel, Belgium), and GE Healthcare (Chalfont St. Giles, UK) consolidate their markets, many applications that have escaped their attention are being developed by smaller, more-nimble players.
Niche Applications. Much of the innovation displayed at the 2006 RSNA meeting came from manufacturers that are developing niche market applications. One of the best-known examples of this niche approach is Hologic Inc. (Bedford, MA), which identified the breast imaging market as an underserved niche market in 1986. Hologic has become a leader in this market, and closed fiscal year 2006 with record revenues of $154.1 million and a product backlog equivalent to $194.7 million. Numerous examples of companies staking out their own niche markets were in evidence at RSNA.

SonoSite (Bothell, WA has found its niche in hand-carried ultrasound.
(click to enlarge)Hand-carried ultrasound vendor SonoSite (Bothell, WA) is another example of an imaging company that has found its niche. The company targets nonspecialist clinicians using ultrasound at the point of care in emergency departments, intensive care units (ICUs), and operating rooms. SonoSite's technology, which is based on valuable intellectual property (IP), serves a growing portion of the ultrasound market that is otherwise underserved by big modality vendors. SonoSite meets all the healthcare portability requirements that many manufacturers miss; its water-resistant units are built to survive drops, can be wiped with harsh disinfectants, and provide wireless connectivity for portable image acquisition. In downtown Toronto, a hand-carried SonoSite system allows one sonographer to provide overnight ultrasound services to three emergency departments. SonoSite has shipped 3000 MicroMaxx ultrasound systems since June 2005, when it released its third-generation mobile system.

An air-cooled, battery-powered (and wireless) portable CT system, the CereTom targets head and neck imaging.
(click to enlarge)Another new modality product on display at RSNA was the CereTom, an air-cooled, battery-powered (and wireless) portable CT system. Manufactured by NeuroLogica (Danvers, MA), the CereTom targets head and neck imaging. Neurology CT studies have traditionally been problematic for many hospitals. Neurology patients are often either trauma cases or acute patients from ICUs. Consequently, neurology exams can be time-consuming and unpredictable, throwing off tight CT schedules and possibly exposing outpatients to distressing scenes of highly acute patients. NeuroLogica received its 510(k) clearance for the CereTom in July 2005 and had shipped 40 systems at the time of RSNA. Identifying neuro imaging as an underserved market segment, NeuroLogica CEO Eric Bailey says, "Only 15% of CT exams are of the head, and the big modality vendors never call on neurologists or neurosurgeons." By creating a portable device that can be operated by physicians, NeuroLogica has created a new market for imaging in ICUs and emergency departments. And, like SonoSite's portable ultrasound, NeuroLogica's portable CT is surrounded by valuable IP.
Another RSNA vendor leveraging a unique product concept is Fonar Corp. (Melville, NY), which manufactures the Upright MRI system. Touted by the company as the only true open MRI, the Upright MRI allows patients to walk right up to the magnet. The system's vertical orientation enables a patient's spine and joints to be imaged in a weight-bearing state. The system's design is also well suited for scanning patients in flexion, extension, rotation, and lateral bending positions. The MRI-compatible motorized patient-handling system can also be oriented horizontally for conventional MRI imaging.
In light of falling reimbursement rates for MRI studies, price pressures have increased substantially, bringing competition among MRI vendors to a fever pitch. In the recent past, Fonar carved out a business selling to smaller freestanding diagnostic imaging centers. Therefore, recent reimbursement changes targeting freestanding centers have affected Fonar's revenues. After a record year in 2005 with $104.9 million in revenue, the company's 2006 revenues fell to $33.1 million, resulting in a net loss of nearly $30 million. During its 28 years in business, Fonar has built a strong patent portfolio in MRI technology that the company has vigorously defended; Fonar has collected $128.7 million from patent disputes with GE Healthcare alone. The Upright MRI is key to Fonar's strategy, and the unique configuration of the system has resulted in sales of the device through GE Healthcare and other large modality vendors. Fonar's recent history of success and challenges is illustrative of the opportunities and risks inherent in pursuing niche product strategies.
Continued Growth. Overall, imaging technology manufacturers at RSNA 2006 demonstrated solid progress, with top vendors largely focused on advancing their existing products. Siemens introduced a new Axiom Aristos FX Plus, which features more-compact positioning with separate detector and tube suspension that moves around the patient, rather than moving the patient. With the number of radio-frequency procedures declining, the company's revised Axiom Luminos TF has added a mobile 14 ¥ 17-in. detector to support both fluoroscopy and radiographic studies. In addition, the Syngo Dyna CT can now provide cross-sectional imaging in an angio suite. Acquired data creates 3-D CT-like images that can detect certain anatomies and help find bleeding during angiography procedures. Siemens also updated both the dBA and dTA bottom- and top-mounted versions of the Axiom Artis angiography suite with digital biplane angiography optimized for neurology studies.
In an apparent competitive response to other hand-carried ultrasound systems, Siemens also featured its Acuson P10 system. This small, lightweight system is intended to serve as an extension of the doctor's stethoscope, providing greater insight into the body while remaining outside of traditional diagnostic imaging departments. With its permanently attached scan head, the P10 is convenient for guiding biopsies and directing the insertion of needles or catheters.
Innovation in the CT imaging market is being driven by technologies that enable physicians to image structures and pathology that could not be imaged with previous technologies. Features such as greater speed and different energy levels bring new visualization capabilities to the market.
At RSNA, Siemens took computed tomography to the next level with its 128-slice Somatom Definition. The system uses two 83-millisecond x-ray tubes, enabling the system to complete a 128-slice study in 164 milliseconds—or a 64-slice study in roughly half the time (83 milliseconds). In cardiology studies, a shorter data acquisition time makes it possible to generate good images at a higher heart rate, a capability that can mean less medication required for patients undergoing cardiac CT exams. The two tubes can also image a patient at different energy levels; the resulting data provide a much greater ability to characterize tissue, which enables the masking of bone structures in neurological or vascular studies.
According to Scott Goodwin, vice president of Siemens's CT division, "The slice race is over in CT; there is no demand for a 256-slice CT."
However, that sentiment would be unlikely to gain approval from Toshiba America Medical Systems (Tustin, CA), which used the 2006 RSNA meeting to show off its 256-slice CT scanner as a works-in-progress technology.
Toshiba is leveraging 256 slices to provide more-accurate images at lower radiation doses, with a system that can scan a brain or a heart in just one rotation. The Toshiba design will also make possible dynamic perfusion studies of major organs. The system uses Toshiba's Quantum detectors, with a matrix of 256 ¥ 0.5-mm slices and 125 cm of organ coverage per rotation. A prototype model has been in operation in Japan, and a beta system is slated to be installed at Johns Hopkins University (Baltimore) in February 2007. Several papers on the technology were presented during the RSNA show, including one on human subjects. Toshiba estimates that a 256-slice scanner is two years from market.
Toshiba also showed another work in progress, a new Excelart Vantage Atlas 1.5T MRI. Using a new 128-element system, this MRI should deliver high-resolution images across the entire body with faster imaging times. The new Atlas system design uses an integrated-coil concept that enables clinicians to perform multiple exams without repositioning the patient.
Another new Toshiba product unveiled at RSNA was the Aplio XG ultrasound system. This high-end diagnostic ultrasound system includes new 4-D technology for the acquisition of volumetric data sets that can subsequently be analyzed offline. Like other manufacturers of high-performance ultrasound systems, Toshiba is using proprietary imaging technologies to up-sell hospitals into buying proprietary workstations (and hopefully image-management systems) in addition to the ultrasound system. Given the mature nature of ultrasound as a diagnostic imaging modality, however, such proprietary features seldom represent must-have diagnostic capabilities.
Philips Medical Systems (Bothell, WA) showed a number of interesting applications of volumetric data in its booth. Philips introduced the iU22 high-end ultrasound system, which uses iSlice, the company's proprietary volumetric data scheme. The iSlice system includes a new 4-D cardiac scan head, the X7-2 x-matrix array, and QLAB workstation.
In another application of 3-D, Tim Mueller, stereolithographer from Nevada Imaging Centers (Las Vegas), showed how the centers employ CT and MRI studies to create 3-D models, which are used by referring physicians for surgical planning and patient education. The models have proven to be effective in differentiating Nevada Imaging Centers' services, and have resulted in a significant increase in exam volumes.
Philips was also leveraging its strength in consumer electronics and lighting with "The Ambient Experience," a series of clinical environments created for the company's modalities.
The Philips Achieva 3.0T MRI received a lot of attention from RSNA attendees. The Achieva 3.0T X series enables clinicians to conduct routine to advanced imaging across all clinical applications. The new Achieva 3.0T X series will be available in a mobile configuration, the first and only one of its kind in the industry. The new system includes an alphabet soup of new features like 2K imaging, 4-D-Trak, sensitivity encoding (SENSE), FiberTrak, SENSE Spectroscopy, and diffusion-weighted whole body imaging with background body-signal suppression (DWIBS). In a bit of creative product roadmapping, Philips Achieva 1.5T XR systems can be upgraded to the 3.0T by replacing the RF coil.
At RSNA 2006, GE Healthcare launched several new systems. The LightSpeed VCT XT volume CT scanner, which targets cardiology imaging, reduces a patient's radiation exposure by up to 70% for diagnostic cardiac scans and is capable of capturing images of the heart and coronary arteries in as few as five heartbeats.
GE also unveiled two new ultrasound systems at RSNA. Addressing the growing demand for real-time imaging at the point of care, the company's Logiq i is a premium system that brings specialized, console-quality imaging performance and laptop-sized portability to serve the general imaging needs of radiology. The second system, the Logiq P5, is a mid-range system, targeting private practices, specialized clinics, and community hospitals. GE has brought advanced high-end imaging capabilities to the P5 that include the company's patented high-definition speckle-reduction imaging (HD-SRI) feature, which heightens visibility of organs and lesions with high-definition contrast resolution that suppresses speckle artifacts while maintaining true tissue architecture. The P5 also supports CrossXBeam spatial compounding, which enhances tissue and border differentiation with a spatial compounding acquisition and processing technique. The product also features 4-D imaging, which acquires 3-D images in real time to reveal the anatomical details.
Overall, RSNA 2006 saw solid progress in the field of imaging—much of it proprietary in nature. The largest vendors leveraged their size and access to capital by advancing technology on big-ticket items. Many of these new offerings compete as much based on their proprietary technology as they do by offering truly superior solutions (see sidebar). Meanwhile, smaller vendors demonstrated their ability to find and quickly capitalize on market opportunities overlooked by larger vendors. Certain products—such as SonoSite's portable ultrasound and NeuroLogica's portable CT—are based on proprietary IP.—Tim Gee, principal, Medical Connectivity Consulting
References
1. The Worldwide Orthopedic Market: 2005– 2006 (Chagrin Falls, OH: Knowledge Enterprises, 2006).
2. Global Orthopedics (New York: Datamonitor, 2006).
3. 2006 Orthopedic Industry Forecast (New York: Healthpoint Capital, 2006).
4. “Boston Scientific Corporation Analyst Meeting,” Boston Scientific Web site, Investor Relations, Webcasts & Presentations [online] (Natick, MA: Boston Scientific, 6 November 2006 [cited 5 January 2006]); available from Internet: http://phx.corporate-ir.net/phoenix.zhtml?c=62272&p=irol-presentations.
5. North American Cardiovascular Devices Market—Investment Analysis and Growth Opportunities (San Antonio, TX: Frost & Sullivan, 2006).
6. U.S. Cardiac Rhythm Management Market (San Antonio, TX: Frost & Sullivan, 2006).
7. "Sealants, Glues, Adhesion Prevention Expecting Double-Digit Growth Innovations in the $1+ Billion Heart Valve Market," MedMarkets (Foothill Ranch, CA: MedMarket Diligence, 2006).
8. U.S. Markets for Heart Valve Devices 2006 (Toronto: Millennium Research Group, 2006).
9. U.S. Markets for Cardiac Assist Devices (Toronto: Millennium Research Group, 2005).
10. The Neurotechnology Industry 2006 Report: Market Analysis and Strategic Investment Guide of the Global Neurological Disease and Psychiatric Illness Markets (San Francisco: NeuroInsights, 2006).
11. "Emerging Treatments for Epilepsy and Seizure Disorders," Neurotech Insights: The Neurotechnology Industry Newsletter 2, no. 6 (July 2006): 1, 9–11.
12. Medical Imaging (Equipment, Agents & Consumables) (Cleveland: Freedonia Group, 2007).
Copyright ©2007 MX
About the Author(s)
You May Also Like