Originally Published MDDI April 2006 ExtrusionOEMs must make sure potential extrusion providers can create tubing with the properties required for a particular medical device application. Mark A. Saab
April 1, 2006
Extrusion
|
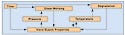
Figure 1. Various interactions take place during the extrusion process. A combination of these interactions can result in material degradation. |
Most specifications for medical tubing consist of a drawing of a tube that lists its material, dimensions, and tolerances. For single-lumen tubing, the dimensions usually include two of the following three dimensions: inner diameter (ID), outer diameter (OD), and tubing wall thickness, along with their associated tolerances. The tube length and tolerance would also be specified unless the tubing is to be provided in a continuous length on a spool. Other notes that may appear on tubing specifications include packaging requirements, a sampling plan for inspection of the dimensional tolerances, and a note regarding tubing cleanliness, such as “No dirt, grease, oil, etc. to be present on the tubing surface.” Very few specifications include other tubing attributes or process parameters associated with producing tubing.
It is a common misconception that as long as tubing is made from the proper material and meets the dimensional requirements, each lot will be the same, regardless of which supplier extrudes it. Although this may be true, there is also a good chance that tubing lots may differ from one another. These differences are not always obvious or easily recognizable, even when inspected by incoming quality control. Often, the process parameters and the equipment used to extrude the tubing are as important as, or even more important than, the dimensions of the tube. Therefore, it is important for both extrusion providers and OEMs to understand the process of extrusion as well as the implications of those parameters for different materials used to make tubing.
Extrusion and Degradation
The process used to produce medical tubing can be extremely important in the high-end diagnostic and therapeutic catheter markets. Market pressures have driven catheter manufacturers to design ever smaller devices with increasingly thinner walls. Examples of tubing applications include high-pressure catheter tubing, tubing used in angioplasty and stent-delivery catheters, and balloon tubing used in medical balloons, especially high-pressure angioplasty and stent-delivery balloons. They also include tubing that will be implanted or inserted in the body and other applications in which the tubing's mechanical, physical, chemical, electrical, or thermal properties are critical to the function of the finished device.
Degradation during extrusion can greatly affect the properties of the end-use medical tubing. Polymers are very large molecules that derive their unique and useful properties from their size, or molecular weight. The process of these large molecules breaking down is called degradation. At some point, polymer degradation changes tubing properties such as tensile strength, brittleness, flexibility, and discoloration. To understand degradation, it is important to understand the various interactions that take place during the extrusion process. Figure 1 provides an overview of these interactions.
Degradation during extrusion can be attributed to a number of causes. Improper drying or overheating the material (i.e., running the polymer at too high a temperature) may cause degradation. Overshearing the material (i.e., running the polymer at too high a screw speed or using the wrong screw design) or keeping the polymer in the molten state too long (i.e., long residence time) may also be to blame. Property changes occur primarily because these factors affect polymers' chemical composition. Some polymers, such as polyethylene terephthalate (PET), are very sensitive to process parameters and can degrade easily, while other polymers, such as polyethylene, are very forgiving. Degradation makes most polymers brittle and reduces the tensile strength and usable life of the finished product.
Another cause of degradation in extrusion is multiple melting process steps. For example, some materials used to make medical tubing must be precompounded. In other words, the base material is melted and mixed with other materials, such as colorants, radiopaque fillers, stabilizers, and processing aids. The precompounding often requires a separate extrusion operation to ensure good dispersion and distribution of the components. This process step results in heat and shear histories, in addition to the heat and shear histories that will be created in the tube extrusion process. If either step is carried out incorrectly, the tubing may degrade.
|
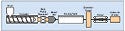
Figure 2. A typical medical tubing extrusion line. |
Extrusion Overview
An extrusion line comprises several pieces of equipment. The major elements of a medical extrusion line include a resin-drying system, an extruder, a die, a cooling tank, a take-up device (puller), and a cutter or winder (see Figure 2).
Drying. Often, the first step in the extrusion process is to dry the polymer. Polymer drying is a critical process in extrusion. Many polymers used in the medical device industry are hygroscopic, meaning they absorb moisture readily from the environment. Hygroscopic polymers must be carefully dried before being melt extruded or compounded.
Different materials require different drying methods, and the temperature at which the material is dried depends on what the material can withstand. Generally, drying temperatures range from 120Þ to 350ÞF, and drying times are 1–4 hours or more. Some materials are extremely sensitive to moisture content and must be dried very carefully. For example, the drying method used with PET is critical to the extrusion process, because any amount of moisture can ruin PET. Others are easier to dry and do not need much oversight.
Drying a material for too short a time or at too low a temperature can result in underdrying. Residual moisture in the polymer can cause hydrolysis during extrusion. Hydrolysis is a degradation process that results in significantly lower molecular weight. Underdrying of polymers often occurs in medical extrusions when processors run multiple materials each day through only one or two dryers. In such cases, the materials will likely not be sufficiently dried, based on the short drying time they would receive. Manufacturers often request the same sizes of tube to be provided in multiple grades or durometers of materials. If the processor does not have three separate dryers available to predry all three materials, then the second and third materials may not be dried properly before extrusion. If that happens, a manufacturer may evaluate partially degraded material and make the wrong choice for the application.
Overdrying is another problem when extruding medical tubing, because many medical extrusion lines run at very low throughputs, or at a low rate of between 1 and 10 pounds per hour. Many commercial resin dryers for medical extruders are oversized. Therefore, the material can stay in the dryer for 24 hours or more. If not properly monitored, materials may become overdried, which can cause thermal degradation in some materials. Many polymers, such as nylon and polycarbonate, are sensitive to overdrying.
Most resin manufacturers specify minimum drying times and temperatures for their materials. These recommendations must be followed very carefully so that materials are dried properly before extrusion. Normally, desiccant-type dryers are used to achieve proper drying. These dryers must be well maintained, cleaned, tested, and calibrated periodically to ensure that they are functioning properly.
The Extruder. An extruder is a melting and pumping machine. It converts solid pellets into a uniform, molten state and forces the material through the die at a constant rate. The frictional heat generated from the mechanical work of the screw and heat conducted from the heated barrel of the extruder melt the material.
Extrusion Die. An extrusion die sits at the end of the extruder and forms the initial shape of the tube. The die is the point at which the polymer exits into a cooling tank. A tubing die typically consists of two major components: a mandrel or tip that forms the tube ID, and a die, or ring, that forms the tube OD. The die and mandrel are contained inside the extrusion head. There are literally dozens of firms that manufacture extrusion heads and tooling, and many extrusion companies have developed their own proprietary head, die, and mandrel designs. The design of these components plays a critical role in the extrusion process and the ability to produce precise dimensions and maintain proper physical properties of the material. The relationship between the die and mandrel dimensions and the finished tube dimensions is referred to as the draw-down ratio (see Figure 3).
|
Figure 3. The extrusion process that creates the draw-down ratio. |
Very-small-diameter medical tubing with very thin walls can be difficult to extrude through a standard extrusion head and die. Often, the viscosity of the materials in the die is so high and the die gap is so small that the operator must increase the polymer temperature. This reduces the viscosity of the material so that the flow through the die is sufficient. But heating up a polymer can dramatically alter its properties.
Many custom extruders overcome the problems of producing tight-tolerance, small-diameter thin-walled tubing by using high draw-down ratios. This significantly improves dimensional tolerances, increases line speed, and makes tooling (dies and mandrels) much easier to fabricate. Unfortunately, running a high draw-down ratio also imparts significant orientation and residual stress and strain in the finished tubing. Such orientation can significantly increase the tensile strength and reduce the elongation of the tubing in the machine direction. It may also reduce the tubing burst pressure because of the loss in hoop strength. The residual stresses from high draw-down ratios can cause problems during subsequent thermal processing and sterilization and in the course of natural or accelerated aging. Thermal processing can release the stresses built in during extrusion, causing the tubing to shrink in length and increase in diameter and wall thickness.
Cooling. The extrusion cooling process is the next critical step. Polymer cooling is important. Significant changes in physical properties and morphological structure can result from different cooling conditions. For example, many polymers are semicrystalline; in other words, they contain amorphous regions and crystalline regions. When the polymer exits the die and cools, rapid cooling and quenching tend to retard crystallization or completely eliminate it. However, slow cooling can cause a higher degree of crystallinity or very-large-crystal formation. In some medical applications, such as balloon manufacturing, the extruded tubing must be amorphous prior to the balloon-forming process. Therefore, it is important to verify that the cooling parameters used will not cause crystallization in the tubing during extrusion.
In other applications, such as PEEK tubing extrusion, the PEEK tubing must be crystallized when extruded. That ensures that the tubing possesses the thermal, physical, and mechanical properties that PEEK is capable of attaining. In materials such as polyethylene and polypropylene, it is desirable for some applications to minimize the crystallinity in the tubing for improved clarity and softness. In other applications, increasing the amount of crystallinity improves stiffness and lubricity.
Most processors cool the polymer in a water-filled cooling tank as it exits the die. This is typically done in free extrusion or through a vacuum sizing tank. However, in both methods, contact with the water in the tank cools the polymer. The water temperature, circulation of the water in the tank, length of the cooling tank, and line speed can all affect the cooling process and thus the physical properties of the resulting tubing.
Water temperature control in the cooling tank is critical in many applications. However, many processors do not use temperature controllers at all; some have crude temperature control of their cooling water. A lack of control can result in significant variations in polymer cooling rate from one lot to another, as well as from the beginning to the end of a lot. Processors that use tap water for cooling can see incoming water temperatures change 30°F or more from season to season. In addition, hot spots can be created in the cooling tank, especially in the area where the polymer first enters. Indeed, proper circulation of the water in the cooling tank is important, even if precise temperature controllers are used. If there is insufficient flow in the water tank, hot spots can develop over time and be unknown to the processor.
Many medical extrusion lines are sold with very small, undersized cooling tanks that may not be well suited for long production runs or for extruding large-diameter or thick-wall tubing, or for extruding small, thin-wall tubing at high line speeds where there is insufficient time in the tank to cool the tube properly. High line speeds or short cooling tanks can result in insufficient residence time in the cooling tank. If the tube exits the extrusion process early and the inside of the tube is still warm, the cooling process may start reversing itself. This means the tube will rewarm itself from the inside out, since the center of the tube was not sufficiently cooled. This cooling reversal can create varying physical properties in the tube.
Extrusion Equipment and Its Importance
It is extremely important that purchasers make sure their tube manufacturer has the expertise and equipment to manufacture a high-end tube for use in medical devices. In the past five years, many industrial extrusion houses have entered the medical extrusion business because they see higher profit margins than are available in industrial applications. However, often these manufacturers have extruders that are too large for the production of tubing used in the medical industry. Using an oversized extruder to make a medical tube can result in very long residence times. In many materials, excessively long residence time will lead to thermal degradation of the polymer.
In addition, some tubing manufacturers use old equipment or equipment that may not be maintained to the standards desired in the device industry. Many older extrusion lines do not have state-of-the-art controls and, therefore, processing temperatures and other parameters can vary widely. Such variation can cause inconsistent thermal history, and as a result, inconsistent properties can occur within a run or from run to run. The same can be true for equipment that is properly designed but not well maintained, or for equipment that is not properly calibrated. For example, a temperature controller on an extrusion line may operate in temperatures ranging from 300Þ to 600ÞF or more. A temperature controller that is off by 1% equates to 5Þ at 500ÞF. If it is off by 5%, that equates to 25Þ at 500ÞF. With some materials, a process change of 10Þ can result in a dramatic difference in tube properties.
Medical tube manufacturers typically have very small extruders. But medical devices often require larger-diameter tubing than these small extruders were actually designed to produce. In these cases, processors may be running the extrusion lines at their maximum output with high screw speeds. This can be detrimental to many polymers that are shear sensitive. Shear-sensitive polymers that are run at a high screw rpm can suffer the same type of degradation found when a polymer is heated for too long or at too high a temperature. It is important to recognize that numerous interactions take place during an extrusion process.
Quality Issues
The OEM should determine what tests it should conduct on incoming tubing, as well as what tests it will specify the processor to conduct. The tests, if any, should be dependent on the end-use requirements of the product.
Any extrusion house that manufacturers choose to partner with should be ISO 9001:2000 or ISO 13485 registered. But ISO registration does not guarantee that high-quality tubing will be produced. Rather, ISO is a quality management system that ensures that a company is operating at some minimum standard. Still, an OEM should investigate an extruder's level of expertise. OEMs should also make sure the company has state-of-the-art manufacturing equipment and highly trained personnel, and they should guarantee that the extruder has in place the appropriate processes for manufacturing the product.
Conclusion
A tube's dimensions can affect the performance characteristics of extruded medical tubing. However, process parameters, equipment, and material characteristics also play an important role in determining the end properties of an extruded tube. When choosing a tubing supplier, OEMs should take into account the requirements of the tube in the finished device. They should also consider how important the tube's performance characteristics are in ensuring the proper device function. Since it is not possible or practical to specify every critical characteristic of a given tube, OEMs should seek out suppliers that have a demonstrated history extruding similar materials in similar sizes that are used in similar applications. The tubing supplier also should have appropriate levels of understanding, process controls, and expertise for the intended tube application and materials.
Mark A Saab is the founder and coowner of Advanced Polymers, Inc. in Salem, NH. He may be contacted by phone at 603-327-0600.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like