The use of devices to treat diseases is often reserved for patients who have failed a few drug therapies. This tradition is partly driven by doctors underestimating the drawbacks of many drugs, as well as a reimbursement system that has a shortsighted time horizon. Some devices, especially as their related procedures become less invasive, should move up in the treatment paradigm because they may provide better long-term clinical outcomes more cost-effectively than drugs.
To help devices earn the respect they deserve, manufacturers should work to develop technology that overcomes early adoption obstacles.
|
Mark Speers |
|
Susan A. Posner |
|
Jennifer Kim Cutie |
Traditional Treatment Paradigms
The typical treatment paradigm treats early-stage disease with drugs, reserving procedures and devices for patients with late-stage disease or for those who do not respond well to or cannot tolerate drug therapy. For example, ophthalmologists treat glaucoma for years with drugs including prostaglandins and beta-blockers. They often resort to dual or triple therapy to keep intraocular pressure (IOP) under control despite well-known difficulties with patient compliance. According to the clinical literature, up to 60% of patients are not compliant with prescribed drug regimens.1,2 Physicians only turn to trabeculectomies and implanted shunts after a patient who has undergone laser treatments continues to experience rising IOP on multiple medications. Likewise, patients suffering from chronic pain are often treated with strong opioids for decades and usually only learn of the option of spinal cord stimulators through their own research.
The initial trial of a drug therapy is the path of least resistance for all three constituencies—patient, physician, and payer. The patient hopes the drug provides a quick fix compared with a frightening procedure involving anesthesia and scalpels that disrupts the patient’s schedule. The physician values the opportunity to conserve precious office time by avoiding the need to sell the patient on the procedure. Also, in situations in which the physician would have to refer the patient to a specialist to perform a procedure, the physician risks losing the patient. Finally, the payer prefers the relatively low cost of drugs for a perceived maximum of 18 months (the average time a health plan member will stay on a current plan) to the greater immediate expense of a procedure.
In general, the initial trial of a drug may be well advised because first-line drugs for many diseases are reasonably efficacious, safe, and often inexpensive, especially when they are generic. However, the instinctive overreliance on drugs leads to subsequent trial-and-error prescribing of increasingly expensive and less-benign drugs. Devices often should explicitly intervene in this paradigm rather than be implicitly relegated to last resort-status.
Evaluating Devices Objectively
Two flawed underlying assumptions drive the traditional treatment paradigm to favor drugs inappropriately. The traditional view assumes drugs are both safer and less expensive than devices. However, all drugs have unintended side effects, some that lead to noncompliance, others to morbidity, and, sometimes, mortality. Because drugs act systemically, many of them have an unsafe nature. Devices, by definition, generally act more locally. For example, recent experience has shown that thoroughly tested drugs like Vioxx and Zelnorm, prescribed for pain and irritable bowel syndrome, were far more dangerous than their deceivingly low dosages would suggest. Although devices can also cause unintended morbidity and mortality, several risks commonly perceived as associated with devices are far lower than might be expected.
For instance, according to the Institute of Medicine, anesthesia care today is nearly 50 times safer than it was 20 years ago, with one anesthesia-
related death per 200,000–300,000 cases. Although the idea of an implanted device may raise concerns of failures or side effects, many devices have shown higher efficacy rates with lower risks than their pharmaceutical counterparts. For instance, current intrauterine devices like Mirena and Paragard are considered >99% effective. In contrast, efficacy estimates for typical use of oral contraceptives range from 92 to 97%. Risks and side effects are also more likely to occur with chronic use of oral contraceptives and may include serious complications like blood clots and hypertension. Like IUDs, many other devices have the added benefit of ensured de facto compliance after treatment, which is different from drugs. Patients are often not compliant with prescriptions for drugs, and unpleasant side effects or inconvenient dosing schedules lead to lost opportunities to adequately control disease progression.
Drug therapy can also prove very expensive. A per-day cost of a chronic drug is often around $3, translating into annual costs of $1100. For a patient with an expected survival of 20 years, this represents a total of $22,000 without inflation. Assuming a 5% annual price increase and applying a 10% discount rate for the annual cost of money, the current value of these 20 years of therapy is $14,600. An economist would argue that an equally safe and efficacious device intervention would be cost-effective for society at any price below $14,600. Many device-versus-drug comparisons therefore represent great values. For example, a $2400 arthroscopic debridement and microfracture procedure to conserve and proliferate cartilage breaks is a great value—even if it can eliminate the use of $3/day painkillers for just three years. Likewise, the implant of a sterilization device such as Essure by Conceptus is reimbursed at approximately $2200, which breaks even for payers if it replaces $3/day oral contraceptives for two years.
The economics are even more compelling in favor of devices in diseases in which drug therapy often culminates in the use of a device, such as the glaucoma example offered earlier. In these situations, 20 years of prostaglandin therapy, including supplemental beta-blockers for the last 10 years, costs more than $12,000 before the cost of the trabeculectomy. On the other hand, investing in the trabeculectomy immediately might avert the need for drugs and only represent a cost of around $2000–$2500.
Unfortunately, health insurers employ shortsighted economic metrics. Knowing that the average member changes insurers every three years, insurers perceive that their economic breakeven has to occur within
18 months on the average member (assuming the average member is halfway through his or her membership tenure when the treatment decision is made).3 Obviously, this shortsighted metric favors drug therapies nearly universally. The arthroscopic and the sterilization procedure options appear terribly expensive with this metric. By relying on this 18-month metric, the insurance industry is systematically incurring more costs nationally for two main reasons. First, the average 18-month tenure is misleading; it is suspected that chronically sick people actually try much harder to keep their plans. Second, in most geographies, there are only a few major insurers, so members cycle back and forth.
Brighter Future for Devices
|
Figure 1. (click to enlarge) Assessment of alternative treatments. Burden is defined as the sum of the cost of the therapy and the combined clinical morbidity and mortality of the disease and the therapy’s unintended side effects. Conditions in the top right quadrant are strong candidates for the use of devices while conditions in the bottom left quadrant are likely to rely heavily on drugs. |
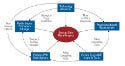
It is likely that devices will move up in the treatment paradigms for many conditions. As examples in Figure 1 show, electrophysiology ablations, bariatric devices, and spinal cord stimulators will migrate upward on the chart. There are at least five reasons why we expect the future to grant devices more respect. The mutual reinforcement of these forces is illustrated in Figure 2 and is explained below.
Devices Will Advance Technically. Miniaturization and improved biomaterials and electronics will create better devices. For example, advances in laparoscopic equipment and materials such as Allergan’s Lap-Band, or potentially even transluminally delivered products will lead to more type 2 diabetic patients receiving procedures rather than relying on multiple drug therapies or drug therapy at all. Likewise, improvements in catheters and energy delivery will enable more patients to receive ablations for chronic atrial fibrillation rather than relying on chronic drug therapy. Solx is developing an intraocular pressure monitor that can be implanted to better monitor and perhaps ultimately control a tunable shunt for glaucoma.
Physicians Will Benefit. Doctors can earn more fees from performing a procedure than from writing prescriptions during office visits. This is particularly true when a device-based procedure can be performed in an office or ambulatory surgery center rather than a hospital operating room. Not surprisingly, we have found that physician specialties trained in procedures are more inclined to recommend procedures and devices earlier in the treatment paradigm. For example, urologists, orthopedists, gynecologists, ENTs, and gastroenterologists are all more inclined to employ devices than their nonsurgical colleagues such as psychiatrists, neurologists, endocrinologists, internists, and rheumatologists. Effective device treatments for severe gastroesophageal reflux disease, sleep apnea, and stress urinary incontinence (such as the recently approved device by Novasys Medical) will benefit from these physician economics.
Health Insurer Economics Will Change. Many of the drugs in development are biologics rather than small molecules. Biologics are inherently more costly to manufacture than traditional small molecules and therefore carry much higher prices. The $3/day benchmark used earlier is but a small fraction of the price of biologics. The rising costs of drugs will make devices more economically attractive. In fact, devices such as those being developed by MicroCHIPS and SurModics may enable more opportunities for local delivery of smaller doses of expensive drugs and may become cost-effective partner technologies. In addition, it is inevitable that insurers will adopt longer time horizons and begin to value device interventions more as their industry consolidates and new federal initiatives seek to provide more continuous coverage. Longer time horizons and drug savings will help home dialysis advances at NxStage and Baxter gain payer adoption.
More Investment in Clinical Trials. As devices increasingly compete against drugs, the burden of proof—both in efficacy and statistical significance—is rising. Medical device executives are therefore committing more of their budgets to clinical trials, both for approval and for coverage decisions after approval. As a striking example, C. R. Bard recently funded a nearly 2000-ICU patient trial of its new endotracheal tube to quantify its impact on the rate of ventilator-associated pneumonia. This type of trial for a 510(k) device is a new phenomenon. Fortunately, the cost of performing these trials is declining as surrogate endpoints are recognized by FDA and as competent clinical research organizations perform the trials in low-cost environments.
|
Figure 2. (click to enlarge) Forces in favor of devices. |
Patient Influence. Patients will seek and demand devices more often as they become better educated about their treatment options and the drawbacks of chronic drugs. The Internet has already greatly improved patients’ access to information. The technological improvements will make the procedures less frightening. In addition, as copays on drug benefits increase, patients’ economics will increasingly favor procedures. A branded drug for a chronic condition can easily require a copay of $50 per month ($600 per year). Meanwhile, a procedure may only cost the $500 deductible of the medical benefit. If the patient has already paid for $300 of the deductible, the effective cost of the procedure is only $200. Compared with drugs, this results in less money spent by the patient. As an example, Candela’s laser for treating acne has benefited from this trend.
Conclusion
The medical device industry has a bright future, particularly if its executives focus on developing products that address and overcome the historic obstacles to the earlier adoption of device interventions within a disease’s progression. Moving up in clinical paradigms will uncover enormous markets for the device industry.
References
1. BL Nordstrom et al., “Persistence and Adherence with Typical Glaucoma Therapy,” American Journal of Ophthalmology 140 (2005): 598–606.
2. B Sleath et al, “Patient-Reported Behavior and Problems in Using Glaucoma Medications,” Ophthal-
mology 113 (2006): 431-436.
3. R Cebul et al, “Employee-Based Insurance Markets and Investments in Health” (preliminary draft), Case Western Reserve University (July 2007).
About the Author(s)
You May Also Like