Originally Published MDDI June 2001Medical Design Excellence Awards 2001
June 1, 2001
Originally Published MDDI June 2001
 The 9 gold and 18 silver award-winning products in the fifth annual MDEA program are each distinguished by their pioneering design and manufacturing achievements. To paraphrase one of this year's jurors, the designers and manufacturers of the products on the following pages sweated the details and paid close attention to industrial design qualities.
The 9 gold and 18 silver award-winning products in the fifth annual MDEA program are each distinguished by their pioneering design and manufacturing achievements. To paraphrase one of this year's jurors, the designers and manufacturers of the products on the following pages sweated the details and paid close attention to industrial design qualities.
Innovative medical devices—particularly those involving concepts that are on the leading edge of modern technology—are often hindered by setbacks. Technologies that ultimately provide significant benefits are sometimes controversial at the outset and must withstand increased scrutiny. Soon after the announcement of this year's MDEA winners, one device was voluntarily removed from the market, and production and sales of the device were halted. In view of the issues that led to this device's removal from the market, the MDEA jurors elected to withdraw that award.
The MDEA program is presented by Canon Communications llc and sponsored by MedSource Technologies, DuPont Tyvek, Agion Technologies LLC, Battelle, Avail, Colorado MEDtech, IBA Medical Sterilization & Analytical Labs, and Medical Device & Diagnostic Industry.
Critical Care and Emergency Products
GOLD WINNER
Departing from the Tried and True
XCalibur mobile transporter 6
Submitted and manufactured by Ferno-Washington Inc. (Wilmington, OH)
 The key to developing the XCaliber mobile transporter was to view the entire process from a new perspective. "One major challenge was the departure from tried-and- true roll-pinned aluminum tube structures," says David R. Linger, director of global product development for Ferno-Washington. "Going with glass-resin pultrusions and plastics was a cultural change at all levels." Taking the product from concept to market required the combined efforts of engineers, designers, prototype technicians, and technical writers. Linger adds, however, that the project "was championed by top management and ownership."
The key to developing the XCaliber mobile transporter was to view the entire process from a new perspective. "One major challenge was the departure from tried-and- true roll-pinned aluminum tube structures," says David R. Linger, director of global product development for Ferno-Washington. "Going with glass-resin pultrusions and plastics was a cultural change at all levels." Taking the product from concept to market required the combined efforts of engineers, designers, prototype technicians, and technical writers. Linger adds, however, that the project "was championed by top management and ownership."
The result of this process was the creation of the first composite ambulance cot in the emergency medical service (EMS) industry. The XCaliber is used by EMS personnel to transport patients to healthcare facilities from diverse sites, including accident scenes.
The cot not only had to be lightweight while capable of bearing a substantial load, but also had to optimize the delivery of prehospital care, withstand outdoor elements, and meet rigorous crash-testing and federal standards. Analysis of marketing data showed two principal needs for a new cot: the capacity to hold larger patients and design features to help reduce the back injuries of emergency medical technicians (EMTs).
To meet these needs, Ferno-Washington chose new lighter and stronger materials: multilayer resin and glass composites and epoxy adhesives. The design team selected a pultrusion process for molding the structural frame of the cot. The use of composite materials also allowed the design team to meet the additional goal of producing an aesthetically simple and cleanable cot, with subassemblies that can be easily replaced by the customer using simple tools.
With its 600-lb capacity, the cot can accommodate very heavy patients—allowing the EMTs to simply raise the cot and roll the patient to, and then into, the ambulance, where the cot is then safely secured in a cot fastener. For very large patients, the team designed an auxiliary large-body-surface patient deck that mounts over the mattress and standard bed surface, and locks securely onto the main frame.
The transporter was designed to provide certain ergonomic benefits to its operators. Gripping surfaces were designed to provide superior grasping positions, and the frame is adjustable at each end to promote proper lifting techniques. The thumb-tab controls allow operators to maintain their grip on the cot while using the controls. The main frame of the transporter is constructed with an elliptical shape that enables additional helpers—who are often untrained volunteers—to grasp the cot easily and with a proper secure grip.
The application of improved ergonomics, the novel use of plastics and other materials, and other key concepts of the design process all required a new approach to product development, according to XCaliber team leader Jeffrey Flynn. "We took every liberty to innovate and still remain within the product specification," he explains. "The product that resulted was exciting because we made up our minds, as a team, to do what had never been done before without letting product history drive our thinking."
The design team also maintained a clear view of the relationship between product designers and consumers. Says Flynn, "I never forgot and I never let my team forget that the consumer is not usually the innovator. It is rare that the consumer says, 'hey, let's invent this thing to do this' and have a milestone product as a result. That's our job." He explains, "A product specification is fine, but it has as much to do with maintaining focus and direction internally as with meeting the needs of the consumer. To innovate, you have to reach out 10 to 15 years and discover what you want, as product designer, to be the mainstay in the market that far in the future."
An essential element in the development of the XCaliber patient transport system was incorporating a degree of unconventional thinking into the design process. Says Jeffrey Flynn, XCaliber team leader, "The challenges our team had to overcome included the 'build everything on the workbench philosophy.' Nothing beats a hands-on model, but refinement happens through iteration." On some aspects of the product, 20 or more iterations were performed on the computer before the design team agreed on form and function, according to Flynn. He explains that "the XCaliber was constrained only by a basic product specification, and any opportunity for major innovation was pursued. We never concerned ourselves with 'the way we've always done it.'" |
SILVER WINNERS
Battlefield Tested
Life Support for Trauma and Transport (LSTAT)
Submitted and manufactured by Integrated Medical Systems Inc. (Signal Hill, CA)
 The developers of the Life Support for Trauma and Transport (LSTAT) system strived to create a "smart" patient platform capable of functioning as a portable, networked intensive-care unit (ICU) and surgical table. Development of the product required approximately five years and was initiated by a brainstorming session involving military and industry representatives, according to Matthew E. Hanson, PhD, vice president for business development at Integrated Medical Systems. "What emerged was the vision of an individualized ICU that was both highly compact and highly capable, and could support an unbroken continuum of care from injury site, through evacuation, definitive care, and recovery," he adds. "In short, a 'trauma pod.'"
The developers of the Life Support for Trauma and Transport (LSTAT) system strived to create a "smart" patient platform capable of functioning as a portable, networked intensive-care unit (ICU) and surgical table. Development of the product required approximately five years and was initiated by a brainstorming session involving military and industry representatives, according to Matthew E. Hanson, PhD, vice president for business development at Integrated Medical Systems. "What emerged was the vision of an individualized ICU that was both highly compact and highly capable, and could support an unbroken continuum of care from injury site, through evacuation, definitive care, and recovery," he adds. "In short, a 'trauma pod.'"
In addition to its 5-in.-thick patient platform, the LSTAT system incorporates a state-of-the-art defibrillator, ventilator, suction, three-channel fluid and drug infusion pump, point-of-care blood chemistry analyzer, and patient monitoring subsystems. Onboard power and oxygen subsystems are also provided. Patient data are available at the bedside on a handheld secondary display with optional wireless capability, over a hospital's clinical information system, and via secure Web sites designed to protect patient privacy.
Development of the LSTAT system required that a modular approach be taken in combining the functions of existing medical devices. Components are removed from their housings to minimize weight and volume, individual power systems are also removed, and inner circuitry is separated from the external controls and displays.
Data generated by devices produced by different manufacturers are ported to a common databus, individual datastreams are time synchronized, and a unified stream is then packaged in Internet protocol format for Etherport communication. The resulting product, says Hanson, "is the world's first FDA-cleared suite of diverse medical, data, and utility subsystems."
"Among the company's competitive advantages is its ability to 'leap frog' itself, to absorb customer feedback and rapidly advance to the next generation," says Matthew E. Hanson, PhD, of Integrated Medical Systems. "The LSTAT system has already evolved through three generations since the first functional prototype, achieving lighter weight, smaller volume, and greater capability." |
Adding Versatility to Pulse Oximetry
Radical Signal-Extraction Technology (SET) pulse oximeter
Submitted and manufactured by Masimo Corp. (Irvine, CA)
 According to Jeff Herbert, I.N. Incorporated sales and marketing manager, among the technical achievements of the Masimo Radical signal-extraction technology (SET) pulse oximeter was "integrating all desired features and specifications into a portable and modular monitor design that maintains comfortable, ergonomic, and portable form-factors, and still being able to meet environmental test requirements." The product is intended to provide continuous noninvasive monitoring of functional oxygen saturation of arterial hemoglobin and pulse rate for adult, pediatric, and neonatal patients in hospitals, hospital-type facilities, and mobile and home environments.
According to Jeff Herbert, I.N. Incorporated sales and marketing manager, among the technical achievements of the Masimo Radical signal-extraction technology (SET) pulse oximeter was "integrating all desired features and specifications into a portable and modular monitor design that maintains comfortable, ergonomic, and portable form-factors, and still being able to meet environmental test requirements." The product is intended to provide continuous noninvasive monitoring of functional oxygen saturation of arterial hemoglobin and pulse rate for adult, pediatric, and neonatal patients in hospitals, hospital-type facilities, and mobile and home environments.
The device design allows the part of the monitor that contains the main pulse oximetry function to be separated from the base station during operation for use as a fully functional handheld monitor. Trending data stays with the handheld device and thus with the patient. A built-in gravity detector automatically rotates the screen orientation when the device is changed from a horizontal to vertical position.
According to Peter Lang, Masimo product manager, "A key design achievement was to incorporate all the diverse user and design requirements into a versatile, innovative product that finds application throughout the hospital and healthcare environment, while breaking the traditional system design limitations so commonly found with medical devices." Lang adds, "The device's SatShare feature allows the Radical to interface and upgrade virtually any existing patient monitors without any additional, expensive interface equipment. With the Radical a hospital can truly standardize on one product and pulse oximetry technology throughout all departments of a hospital."
SUPPLIER FILE: I.N. Incorporated (Los Alamitos, CA)
Dental Instruments, Equipment, and Supplies
GOLD WINNER
Promoting Better Oral Hygiene
WaterPik Flosser
Submitted by Volan Design (Boulder, CO); manufactured by WaterPik Technologies (Ft. Collins, CO)
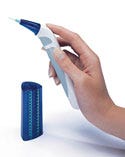 This device does the unlikely," says MDEA juror Michael Wiklund. "It takes a task that most people hate and makes it fun—almost something to look forward to." Juror Eliot S. Lazar adds, "From a practical standpoint, it has a vast audience and a wide range of utility that may have a beneficial impact on a broad segment of the population."
This device does the unlikely," says MDEA juror Michael Wiklund. "It takes a task that most people hate and makes it fun—almost something to look forward to." Juror Eliot S. Lazar adds, "From a practical standpoint, it has a vast audience and a wide range of utility that may have a beneficial impact on a broad segment of the population."
According to Wiklund, "The jurors who tried it liked it and would have written a check on the spot for the product. That is evidence enough that the product is a winner, not only from a design standpoint, but from a marketability standpoint."
The Flosser is a lightweight, handheld device that is highly portable and easy to use. It was designed to address the 90% of the population that know they should floss but just don't do it. The filament action gently reaches between teeth and below the gum line to remove the plaque that is associated with causing the gum disease gingivitis.
The primary goals for the WaterPik Flosser were to create an ergonomically comfortable product that would promote periodontal care and would be easier to use than conventional floss. It had to be aesthetically consistent with typical bathroom decor and have a minimal footprint.
To accomplish these goals, the designers of the WaterPik Flosser incorporated a number of novel ergonomic concepts. According to Wendy T. Volan of Volan Design, "The designers deliberately angled the flosser at the tip to facilitate interdental insertion, carefully positioning the single control button on the neck of the unit and adding a soft-touch, rubberized grip for increased ease of handling."
The ergonomic form of the device evolved as an extension of the fingertip for ease of aiming the tip when it is in the mouth and out of view. This allows the tip to be used comfortably in the areas where plaque typically forms.
The Flosser's tip was specifically engineered to be flexible enough to provide a whiplike action without irritating the gums. In addition, the designers note that the tip can never break off between the teeth. The styling of the Flosser handle incorporates a gentle "s" curve into the parting line that, when set on the counter, keeps the device from rolling over and the tip from touching the countertop. The soft-touch surfaces add color and style while enabling users to maintain their grip even with wet hands.
The vibrating tip generates 10,000 strokes per minute, enabling the single-use nylon filament to clean and strengthen gums gently. It is intended for use by anyone age eight and up, including those with special oral healthcare needs such as braces, crowns, or bridges.
Says Volan, "The next generation of the initial Flosser is a rechargeable version. The battery-powered version was redesigned to accommodate the extra space needed for the recharging electronics." The unit consists of a soft-grip handle that accepts a single AA battery, a cartridge containing a month's supply of replaceable flossing tips, and a stand or charging base, depending upon model. Also available is a set of color-coded, snap-on hygienic sleeves so that multiple family members can share the device without sharing germs.
The development process used an "art-to-part" methodology in that all design information was generated and communicated digitally throughout the entire process, reducing rework and time to market. The original design definition data were used to develop the industrial design surfaces and renderings, engineering documentation, and a prototype for clinical evaluation, as well as for direct machining of preproduction samples, and finally for full-production injection-molded tooling.
According to Volan, the 12-month development process for the product required a cooperative effort by design team members. She explains that "a cross-functional product development team was assembled with members of Volan Design and WaterPik Technologies. Skill sets bridged marketing, engineering, industrial design, oral-care clinical studies, marketing research, manufacturing, and sales." Volan adds that "tight integration of the in-house and design teams resulted in goals and schedules being met."
"Ergonomics were the biggest challenge for this product," according to Wendy T. Volan. "Perhaps the most important aspect of the product design was that it should be intuitive to use and easy to maneuver for everyone—from preteens to senior citizens. Ease of use was considered fundamental to widespread product acceptance." She explains that the design team analyzed the ergonomics of flossing in detail. "The firm ultimately designed the lightweight, compact unit to act as an extension of the hand, capable of being manipulated with subtle fingertip control." Volan Design senior industrial designer Bill Stephens adds, "We designed the device in such a way that inserting the tip into your mouth is a completely natural movement. The shape of the Flosser is designed to act as an extension of your fingertip, making it simple and intuitive." |
SILVER WINNER
Highlighting Whiter Teeth
BriteSmile 2000
Submitted and designed by IDEO Chicago (Evanston, IL); manufactured by BriteSmile Inc. (Walnut Creek, CA)
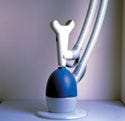 The design concepts behind the BriteSmile 2000 were focused on creating a patient-friendly, nonthreatening system that uses light and a proprietary gel for teeth cleaning and whitening. Care was taken to give the device a nontechnical presence for customers who may be anxious about traditional dental methods. According to John Warner, product manager for BriteSmile Inc., the principal challenges involved mechanical design, electro-optical design, chemistry, and clinical research. Says Warner, "The time frame was about six months. As a start-up company, it's important to test the business concept as quickly as possible."
The design concepts behind the BriteSmile 2000 were focused on creating a patient-friendly, nonthreatening system that uses light and a proprietary gel for teeth cleaning and whitening. Care was taken to give the device a nontechnical presence for customers who may be anxious about traditional dental methods. According to John Warner, product manager for BriteSmile Inc., the principal challenges involved mechanical design, electro-optical design, chemistry, and clinical research. Says Warner, "The time frame was about six months. As a start-up company, it's important to test the business concept as quickly as possible."
The designers chose to use fiber optics carried inside an articulating arm to bring light from the device cabinet to the customer's mouth. At the end of the arm is a light head containing customized emitters that shine light in a constrained pattern on the customer's teeth. The use of multiple emitters to illuminate all of the "smile teeth" at the same time in order to reduce the whitening time to one hour is innovative.
There are three cone-clutch style hinges on the arm that are at rest in the locked position. To move the arm, the operator takes hold of the light head. The operator's hand is then automatically in the correct position to depress the activation switch. This simultaneously releases all three hinges by using air to force the locking mechanism free. Once the arm is in the desired position, the operator lets go of the light head, thereby releasing the switch, and the arm is again locked in position.
A pivot at the base allows the arm to be swiveled aside during gel changes and then brought back into the proper position without readjusting the hinges. It has the added benefit of reassuring patients that they are not trapped between the dental chair and the whitening device, since it can be easily swiveled aside if necessary.
Finished Packaging
GOLD WINNER
Extending the Shelf Life of TPNs
Kabiven multichamber parenteral nutrition packaging system
Submitted and manufactured by Fresenius Kabi (Uppsala, Sweden)
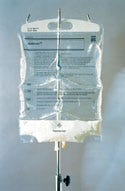 The clinical objective of parenteral nutrition products is to administer appropriate levels of lipids, amino acids, glucose, and electrolytes. Traditionally, such nutritional solutions for parenteral use have been packaged in separate glass containers and administered individually. Fresenius Kabi notes, however, that many hospitals had expressed a need for more-convenient, less-time-consuming methods for administering parenteral nutrition. Lena Soderstrom, the firm's director of project management, adds, "Compared with glass, plastic is lighter, flexible, and resilient to additional knocks. The current trend is toward total nutrient admixtures (TNA) and giving total parenteral nutrition (TPN) from one container made from plastic."
The clinical objective of parenteral nutrition products is to administer appropriate levels of lipids, amino acids, glucose, and electrolytes. Traditionally, such nutritional solutions for parenteral use have been packaged in separate glass containers and administered individually. Fresenius Kabi notes, however, that many hospitals had expressed a need for more-convenient, less-time-consuming methods for administering parenteral nutrition. Lena Soderstrom, the firm's director of project management, adds, "Compared with glass, plastic is lighter, flexible, and resilient to additional knocks. The current trend is toward total nutrient admixtures (TNA) and giving total parenteral nutrition (TPN) from one container made from plastic."
TNA is already a routine procedure in many hospitals and offers a number of benefits; however, it also involves consideration of such issues as metabolic efficacy, stability, and compatibility, as well as the risk of microbiological contamination during preparation and administration. TNA mixes have certain limitations; some must be mixed using a transfer set before administration, others have a relatively short shelf life and must be stored in refrigerated conditions until use.
The main design and engineering challenge of this project was to overcome the limitation of stability. Soderstrom adds, "to produce a product in an overwrap and then to do a final sterilization, and also to find a 'global' composition is a challenge." The path chosen for a portion of this challenge was to design a single package that holds the three TNA solutions separated by peelable seals. This optimizes the quality and the stability of the products while reducing the requirements for controlled handling and storage as compared with mixing a TNA.
The peelable seals make it possible for the end-user to mix the product just prior to use. Hospital mixing of individual solutions for TNA requires aseptic handling that, in order to obtain appropriate safety with respect to microbiological contamination, is time- and resource-consuming. Placing all nutrients in a single closed entity with separate chambers that allows mixing without exposing the solutions to the environment eliminates this work.
The system consists of a three-chamber inner bag enclosed by a cover pouch, both made of polyolefin polymers. The printed inner bag is filled with glucose, amino acids, and lipid emulsion in the three separate chambers. Peelable seals separate each chamber. At the time of use, the cover wrap is removed by tearing at a notch in the plastic cover and pulling it open. The cover wrap and an oxygen absorber, placed between the inner and outer bag to consume any traces of oxygen, are then discarded. The top peelable seal between the glucose and amino acid chamber is opened first. Gripping the sides of the bag above the middle of the seal and pulling the sides outward and downward in a circular movement using pressure from thumbs and index fingers opens the top seal. The bottom seal is opened using the same technique.
Alternatively, the bag can be placed on a flat surface and rolled open starting from the handle side. This opening technique could also be used with the overwrap still on. To get a homogeneous admixture, the bag should be inverted several times after the peelable seals have been opened before start of infusion. In short, to commence usage, the seals are opened, transforming the multichamber bag into a single compartment.
The overall packaging concept was developed for minimal environmental impact by choosing recyclable materials (no PVC or aluminum) that do not pose a risk of toxic emissions or by-products at disposal and waste handling. In addition, the waste volume is minimized, which is important in handling, transport, and landfill disposal. If the container is incinerated, the inherent energy can be reclaimed, according to the manufacturer.
According to Soderstrom, "We started with the packaging concept during 1995, the first stability batches were manufactured by the R&D department in 1996, and the first batches were produced for the market during 1999." The product has received approval for marketing throughout the European Community (EC) countries. Soderstrom notes that several improvements in the original design concept are already being made. "During this year," Soderstrom explains, "we have already made some bag enhancements. For example, we now produce our bags with premanufactured 'holes' in the 'welding shoulders' so that it is easy to hang the bag upside down, which is sometimes desired for the use of special additives."
Lena Soderstrom explains that Kabimix has to be stored under refrigerated conditions before use, and has a rather short shelf life. "The challenge, of course, was to find a more convenient way to package these nutrients, and better methods to administer them." She adds, "The main design and engineering challenge was to overcome the limitation of stability. With this in mind, we developed Kabiven, a three-chamber bag that can be stored at room temperature for 24 months." |
SILVER WINNER
All-in-One Dental Adhesive Package
Prompt L-Pop
Submitted and manufactured by ESPE Dental AG (Seefeld, Germany)
 The Prompt L-Pop is intended to provide an innovative tool to help dental professionals in bonding composite filling materials and fissure sealants to dentin and enamel. Says Oliver Frey, of ESPE, "The basic challenges to overcome in the development process were to develop a chemistry that would work reliably on enamel and dentin at the same time, to design a package that is simple to use although it contains two components, and to stay on time with the schedule."
The Prompt L-Pop is intended to provide an innovative tool to help dental professionals in bonding composite filling materials and fissure sealants to dentin and enamel. Says Oliver Frey, of ESPE, "The basic challenges to overcome in the development process were to develop a chemistry that would work reliably on enamel and dentin at the same time, to design a package that is simple to use although it contains two components, and to stay on time with the schedule."
The single-use package is divided into three compartments. The first compartment contains photopolymerizable methacrylates that are ester derivatives of phosphoric acid, a photoinitiator, and stabilizers. The second compartment contains water, stabilizers, and a complex fluoride salt. The third compartment contains the tip of a microbrush used to apply the filling material and sealant.
The package manufacturing process starts with vacuum molding the compartments into a special foil blister that is composed of three different layers, including polyethylene terephthalate, aluminum, and polyethylene terephthalate. This is followed by filling the first and second compartments with the components described above. The first foil blister is then covered with a second foil layer, which is sealed under temperature. The final production step is the insertion of the microbrush into the third compartment of the package.
Frey comments that "the package's key achievement, from our point of view, is the innovative design that is efficient and fun to use. In fact, the design is reminiscent of a lollipop—that's why the product name: Prompt L-Pop." The firm is currently redesigning the package slightly so the design can be applied to other products, he adds.
General Hospital Devices and Therapeutic Products
GOLD WINNER
Creating a Patient-Friendly Treatment for Lung Disease Symptoms
Acapella chest physical therapy device
Submitted by Product Genesis Inc. (Cambridge, MA); manufactured by DHD Healthcare (Wampsville, NY)
 Acapella is a chest physical therapy (CPT) device that uses an innovative vibratory positive-pressure (PEP) therapy system, combining the benefits of PEP therapy and airway vibrations to mobilize pulmonary secretions. The product is intended for use by patients with lung disease and associated secretory problems, such as chronic obstructive pulmonary disease, asthma, and cystic fibrosis. Regular use of the device can significantly improve the clearance of secretions in a patient's lungs.
Acapella is a chest physical therapy (CPT) device that uses an innovative vibratory positive-pressure (PEP) therapy system, combining the benefits of PEP therapy and airway vibrations to mobilize pulmonary secretions. The product is intended for use by patients with lung disease and associated secretory problems, such as chronic obstructive pulmonary disease, asthma, and cystic fibrosis. Regular use of the device can significantly improve the clearance of secretions in a patient's lungs.
Chloe C. Beirne, marketing specialist with Product Genesis, says "the product's key attribute is its ability to deliver treatment to patients with severe lung disease who are unable to move from the reclining position." Beirne adds, "Additionally, existing systems required the patient to be slapped on the back to mobilize secretion from the lungs. The Acapella's innovative 'rocking' mechanism allows the patient to remove the lung secretion independently, without the help of another person."
The device is considered to be easier to tolerate than other CPT systems, takes less than half the time of conventional CPT sessions, and facilitates opening of airways in patients. There are two varieties to help customize a patient's treatment based on clinical needs, and each device is easily adjustable in terms of frequency and flow resistance by turning an adjustment dial. The device can be used in virtually any spatial orientation. Patients are free to sit, stand, or recline, according to the firm.
The entire product is made using an injection molding process. The impact-resistant device is composed of two primary acrylic plastic materials: glass-filled polypropylene and K-Resin styrene butadiene copolymer. The material selection process was principally based on the manufacturer's key objectives for the product. The material used for the interior components is rigid enough to meet the designers' specified dimensional and density requirements. The manufacturer also wanted the patient to feel comfortable holding and operating the device, so the designers developed a grip-texture for the product. This empowers the patient to feel in control and confident while using the device.
Initially, DHD's key objectives were to develop a vibratory PEP therapy system that functioned independently of gravity controls and met the needs of patients with low-pressure and low-flow constraints. Acapella successfully accomplished both of these objectives and has surpassed projected sales expectations this year.
The manufacturer challenged the design team to develop a device that met the needs of patients with severe respiratory problems, which required the device to be activated at flow rates as low as 5 L/min. The project engineers' solution was a "rocker" system in which a tiny magnet is used to stimulate the vibration needed to mobilize pulmonary sucretions.
Says Beirne, "They placed the system into two different models, one that functioned at flow rates less than 15 L/min and the other at flow rates greater than 15 L/min. Both products have a dial, which allows the user to adjust the resistance and frequency of vibrations to meet their individual needs." Beirne adds that "the product design ensured that patients could receive treatment in any position without the constraints of gravity."
The device is versatile enough to accommodate the needs of lung-disease patients with varying degrees of severity; it can be used with a standard mouthpiece or a mask. It can also be used with a TheraPEP pressure port and gauge for precise pressure measurements, if visual feedback is desired. Finally, it contains a one-way inspiratory valve that enables the patient to inhale and exhale without removing the product from his or her mouth.
"Our firm and DHD Healthcare worked as an integrated team to reduce the product's time to market," says Chloe C. Beirne, marketing specialist with Product Genesis. "The product's functionality was also perfected to ensure that it would be well received in the marketplace." Beirne explains that the device's "soft curves, roundness, colorful appearance, and lightweight body are all intended to communicate a nonthreatening, friendly experience for the patient. The designers were able to incorporate these characteristics in the device's design with the plastics used in the manufacturing process." |
GOLD WINNER
Rapid Prototyping Facilitated Device Design
Protectiv Acuvance IV safety catheter
Submitted and manufactured by Ethicon Endo-Surgery Vascular Access (Cincinnati), a Johnson & Johnson company
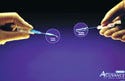 For more than a dozen years, healthcare providers have attempted to address the problem of accidental needlesticks and the resulting exposure of workers to bloodborne diseases. Universal precautions guidelines and OSHA regulations addressed certain elements of the problem, and device manufacturers attempted to develop safer needle designs and sharps containers that offered greater security.
For more than a dozen years, healthcare providers have attempted to address the problem of accidental needlesticks and the resulting exposure of workers to bloodborne diseases. Universal precautions guidelines and OSHA regulations addressed certain elements of the problem, and device manufacturers attempted to develop safer needle designs and sharps containers that offered greater security.
Most of these products, however, have required users to perform specific actions to ensure their safe use —often so complex that workers must undergo training in use of the device. More recently, manufacturers have attempted to create vascular access products that incorporate passive safety technologies—mechanisms that help safeguard workers without the need for special training or additional procedures.
The Protectiv Acuvance IV safety catheter is a sterile, nonpyrogenic, nontoxic, single-use IV catheter device with a safety feature for insertion into a vein and for the administration of medically prescribed fluids. The device has unique design and engineering features: it offers protection against accidental needle-stick injuries, is the first totally passive safety IV catheter, is intuitive to users, and requires little or no training.
The Protectiv Acuvance device is almost the same size as conventional IV catheters. Moreover, the device has a unique needle-holder design to facilitate handling during insertion and threading into the vein. The insertion procedures for the device are identical to those for conventional catheters; there are no changes in insertion technique. The manufacturer notes that, in addition, the hub and safety mechanism design enable the device to be redirected to access the vein if needed. Once the introducer is removed, however, the needle becomes blunt.
The company notes that the main benefit of using the Protectiv Acuvance IV safety catheter is the reduction of needle-stick injuries, protecting healthcare professionals against bloodborne pathogens. In a study conducted in January 1999, a total of 500 device samples were evaluated by 50 clinicians under simulated clinical conditions. The results indicated that there were no safety mechanism failures or needle-stick injuries. The clinicians also commented that the devices were easy to use and rated them as "highly acceptable."
According to Joseph J. Chang, PhD, director of technology at Ethicon Endo-Surgery, "Significant efforts were made in the initial design stage to address customer requirements through a quality function deployment process and manufacturability requirements through design for manufacturability activities. Moreover, the design and development of the Protectiv Acuvance IV safety catheter used state-of-the-art rapid prototype technology for fabricating and critiquing the prototypes." Chang adds, "These tasks significantly reduced the time required to finalize, verify, and validate the design and manufacturing process for speed to market."
In discussing the award-winning product, the MDEA jurors noted that the catheter design represents "a significant improvement over current technology." They added that the key element is that the safety-related feature functions automatically—without the need for intervention by the user, and with no additional operational steps or user training.
SUPPLIER FILE: BioPlexus Inc. (Tolland, CT)
General Hospital Devices and Therapeutic Products
SILVER WINNERS
Simplifying Bone Marrow Sampling
Goldenberg Snarecoil needle
Submitted and manufactured by Ranfac Corp. (Avon, MA)
 Collection of bone marrow specimens is necessary to aid in the diagnosis and treatment planning for patients suffering from various blood disorders such as leukemia, Hodgkin's disease, hypoplasia, and myeloma.
Collection of bone marrow specimens is necessary to aid in the diagnosis and treatment planning for patients suffering from various blood disorders such as leukemia, Hodgkin's disease, hypoplasia, and myeloma.
The Goldenberg Snarecoil needle is a sterile, disposable, single-use device that is intended to provide certain benefits to patients and advantages for clinicians when used to obtain bone marrow specimens. Typically, this device would be used by a physician in an outpatient setting with the patient under local anesthesia.
Basically, the Goldenberg Snarecoil needle consists of two major sections, the handle (proximal) and the needle (distal). The handle is constructed of injection-molded polycarbonate components. The needle is constructed of stainless-steel tubing (cannulae) and wire (stylet) that has been cut, welded, and sharpened. The inner cannula incorporates a snare that has been laser cut. These sections are bonded by a thermal pressing process. The completed device is packaged in a Tyvek pouch and sterilized with EtO.
This innovative instrument features an inner snare mechanism that allows the physician to capture the bone marrow specimen after insertion with the simple movement of its lever. Because the physician does not need to be concerned with severing, distorting, or losing the bone marrow specimen, the Goldenberg Snarecoil can be removed from the patient with a minimal amount of force.
Because the needle does not need to be twisted to sever the sample, the need for additional collection passes will be reduced, the firm suggests, resulting in a considerable reduction in patient pain and anxiety. Moreover, there is a significantly higher bone marrow specimen-retrieval rate. Finally, pathological interpretation is improved because the specimens are longer and less distorted, according to the manufacturer.
Challenging History
Grab 'n Go III portable medical oxygen system
Submitted by Praxair Inc. (Danbury, CT); manufactured by Western Medica, a Scott Fetzer company (Westlake, OH)
 Oftentimes, new technologies meet daunting hurdles of conventionality. Says Praxair's Andrea J. Nicoll, "An opportunity was seen to provide this simplified device to eliminate age-old problems associated with supplying transport oxygen within hospitals." In the past, to provide oxygen for patient transport or mobility in a timely manner, a separate regulator, flowmeter, cylinder wrench, and washer were required. Intended for use by nurses and other healthcare practitioners, the new system is designed to simplify the administration of portable medical oxygen while improving user safety through the elimination of exposure to high-pressure gas connections.
Oftentimes, new technologies meet daunting hurdles of conventionality. Says Praxair's Andrea J. Nicoll, "An opportunity was seen to provide this simplified device to eliminate age-old problems associated with supplying transport oxygen within hospitals." In the past, to provide oxygen for patient transport or mobility in a timely manner, a separate regulator, flowmeter, cylinder wrench, and washer were required. Intended for use by nurses and other healthcare practitioners, the new system is designed to simplify the administration of portable medical oxygen while improving user safety through the elimination of exposure to high-pressure gas connections.
The Grab 'n Go III portable system simplifies medical oxygen use by combining an oxygen cylinder with a regulator and built-in content gauge. Locating these parts and fitting them together properly had been time-consuming. The oxygen regulator and pressure gauge are are now permanently attached to the gas cylinder as a single, integrated unit.
Not only does the integrated design of the Grab 'n Go III oxygen system provide value through ease of use and safety, it also reduces overall cost to the end-user and practitioner, according to the developers.
The system did pose a number of design challenges, according to Praxair's Nicoll. "The critical and most difficult phase of this project was market acceptance. We were challenging 50 years of history that we learned would not easily be overcome."
SUPPLIERS FILE: Lewellyn Design Inc. (Wooster, OH)
Implant and Tissue-Replacement Products
SILVER WINNERS
Performing a Mechanical Ballet
HELEX septal occluder
Submitted and manufactured by W. L. Gore and Associates (Flagstaff, AZ)
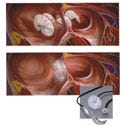 In simple terms, the HELEX septal occluder is a permanently implanted prosthesis indicated for transcatheter closure of atrial septal defects. Pediatric and adult interventional cardiologists use the device as a minimally invasive alternative to open-heart surgery. According to MDEA juror Michael Wiklund, however, "the task of threading the device through the hole, then releasing a spiraling mechanism to seal the hole is a mechanical ballet."
In simple terms, the HELEX septal occluder is a permanently implanted prosthesis indicated for transcatheter closure of atrial septal defects. Pediatric and adult interventional cardiologists use the device as a minimally invasive alternative to open-heart surgery. According to MDEA juror Michael Wiklund, however, "the task of threading the device through the hole, then releasing a spiraling mechanism to seal the hole is a mechanical ballet."
The HELEX is composed of an implantable septal occluder, premounted on a delivery system. The delivery system incorporates a preshaped catheter supplied with radiopaque markers for fluoroscopic visualization during deployment. The basis for the occluder design is a helically shaped wire frame that is elongated to an approximately linear configuration for loading and deployment. The wire frame provides perimeter support for the circular device.
A leaflet that is attached to the support frame and gathered about the center acts to occlude the defect and encourage rapid in-growth of new tissue. An integral locking feature holds the opposing disks together by capturing the three integral eyelets of the support frame. After the device has been deployed, it forms a seal on each side of the atrial defect and the lock encourages each disk to follow the anatomical shape of the atrial septum.
According to Ed Shaw, of W. L Gore and Associates, "The most significant challenge was to incorporate the ability to reposition or retrieve the device in the event of a suboptimal placement. That requirement drove the design of the device and delivery system."
Says Ed Shaw, of W. L Gore, "The strength of the product is its elegant simplicity. The form and function are well suited to the application as emphasized by the common phrase: 'why didn't I think of that.'" |
An Elegant Solution for the Hearing Impaired
Nucleus 24 Contour
Submitted and manufactured by Cochlear Ltd. (Sydney, Australia)
 The Nucleus 24 Contour is a cochlear implant with an electrode array that safely places 22 stimulating electrodes adjacent to the inner wall of the cochlea without the use of invasive bands or positioners. The system is designed to provide useful hearing to people with severe to profound hearing loss.
The Nucleus 24 Contour is a cochlear implant with an electrode array that safely places 22 stimulating electrodes adjacent to the inner wall of the cochlea without the use of invasive bands or positioners. The system is designed to provide useful hearing to people with severe to profound hearing loss.
The implant operates by receiving sound encoded in a radio-frequency link, sent from a speech processor that is worn externally. Next, the implant decodes the information into electrical impulses that activate any of the 22 electrodes on the array to stimulate the underlying nerve cells. The resulting patterns of stimulation are perceived as sound and speech by the user.
"The important aspect of the design of the electrode and delivery system," says Herbert Voigt, MDEA juror, "was the attention to the problem of getting a charge flow at one electrode site to a restricted region of the cochlea." Voigt believes that it is vital if you want to have independent channels of information delivered to the auditory nerves to get the electrode sites as close as possible to those nerve fibers. This has to be done after winding the electrode array through several coils of the snail-shaped cochlea. He adds, "This was a complex design solution that appears to address the major problems confronting cochlear electrode design and insertion."
Peter Gibson, senior project manager for Cochlear Ltd., notes, "One of the great strengths of the Contour electrode is that it is a platform for a wide variety of future enhancements that are currently being pursued by the design team. One such exciting avenue for enhancement is the use of the lumen as a passage for drug delivery."
"The design goals were well known from the start—safety, performance, and ease of surgery," says Peter Gibson, Cochlear's senior project manager. "It is an elegant solution that works." |
Go to the second half of this article
Copyright ©2001 Medical Device & Diagnostic Industry
You May Also Like


