May 20, 2004
Originally Published MPMNMay 2004
SPECIAL SECTION
MDEA: Excellent Partnerships Yield Excellent Products
|
Each year, the Medical Design Excellence Awards (MDEA) recognize outstanding achievements in healthcare product and packaging design. The program, organized byMPMN's publisher Canon Communications, is now in its seventh year. It brings together a panel of judges from across the medical spectrum to evaluate the innovation, design, engineering, user benefit, cost-effectiveness, and other features of each submission. The best are honored with gold and silver awards.
To create these superior products, many of the winning OEMs sought out superior partners. Ranging from small boutique companies to large outsourcing firms with a global reach, these allies offered the supply and design support that led to success. Several also offered their thoughts on conditions that lead to fruitful collaborations, challenges the industry is facing, and trends that will shape its future. --Christina Elston
'Us' and 'them'
|
The Syndeo PCA syringe pump from Baxter Healthcare Corp. |
"Working with partners may have its ups and downs, but that's not necessarily a bad thing," says Tom Black, vice president of OEM sales and marketing at B. Braun Medical Inc. (Plymouth, MN). B. Braun was involved with the System 100 fluid-removal system for complete heart-failure patients from CHF Solutions Inc. (Brooklyn Park, MN). "A successful relationship between a manufacturer and a supplier requires shared experiences, both good and bad," Black says. "I don't think either side gets a trusting feel for the other until they work through a project."
|
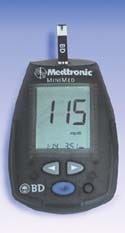
The Paradigm Link untethered wireless glucose meter. |
Trust between OEMs and design partners can even affect a product's success. "Involvement of the manufacturer's internal staff in all phases of the development process, from conceptualization through prototyping, is essential to ensure acceptance of the resulting product," insists Charles Keene, senior vice president of design, Herbst LaZar Bell Inc. (HLB; Chicago). "It is amazing how devastating the 'not invented here' syndrome can be to the successful launch of a new product."
HLB helped design two of this year's award winners. The Syndeo PCA syringe pump, from Baxter Healthcare Corp. (Round Lake, IL), allows patients to control automated delivery of analgesic, sedative, and anesthetic solutions. The Paradigm Link blood glucose meter, developed by BD Medical, Diabetes Care (Franklin Lakes, NJ), Medtronic MiniMed (Northridge, CA), and Nova Biomedical (Waltham, MA), and manufactured for BD Medical, Diabetes Care and Medtronic MiniMed by Nova Biomedical, is an untethered wireless glucose meter that provides bidirectional communication between a glucose meter and insulin pump.
In working with Swiss Medical Care (Lausanne, Switzerland) on the CT Exprés advanced contrast-media-delivery system, Willem van den Bruinhorst, managing director of Medisize Development & Manufacturing (Letterkenny, Co. Donegal, Ireland), says his company's goal was to be viewed as a partner rather than a supplier. "There must be evidence of a partnership culture. The contract manufacturer should be viewed nearly as another department within the OEM. This can be achieved primarily through a teamwork ethos and optimum communication avenues," he says.
Can you hear me now?
|
The Cardiac CryoAblation catheter and CryoConsole from CryoCath Technologies Inc. |
Sometimes this communication has to be maintained despite long distances. During development of the System 100, there were technical contributors in three or more distant cities, as well as the CHF home base, according to circuit designer Ed Merrick of Indulgent Technologies (Stow, MA). He credits the coordination efforts of John O'Mahony of CHF, and O'Mahony's exceptional understanding of the design process, with keeping communication flowing. "He was able to keep each of us well informed about the progress of the project while not bothering us with diversions," says Merrick. "This was a model as to how future designs will be accomplished."
TriVirix International Inc. (Belfast, Northern Ireland, UK), which contract manufactures the System 100 at its facility in Northern Ireland, made sure information about the product's usefulness reached the manufacturing floor. Joshua Rose, director of marketing, says TriVirix routinely requests information from customers on how their products will be used. "We provide that to the people on the floor who are building the product. They understand that they are making a unit that is going to potentially save, and definitely improve, the lives of patients," says Rose.
Both Morelli Designers and CryoCath Technologies Inc. are based in Montreal, but communication was still essential. When Morelli Designers president Michel Morelli and his team were repackaging the design of the CryoCath Cardiac CryoAblation catheter and CryoConsole, CryoCath took an uncommon amount of care in describing its objectives at the outset. "We had a good comprehension of the work we had to do," says Morelli. "We understood the target, and CryoCath understood our design process as well. When there was a question, we had a very clear answer quite fast."
The little things
|
The Avalon CTS cordless fetal transducer system from Philips Medical Systems. |
Understanding that even a product's less-technical features are important, and finding a partner that specializes in those features, can mean a product that better serves customers. "Even the things you don't think of are important in a healthcare-delivery system," says Gary LaTorraca, vice president of MJL Engineering and Manufacturing Inc. (Escondido, CA). Even as CHF Solutions was developing the System 100, MJL was developing a cart to go with it.
LaTorraca credits CHF for recognizing that it wasn't enough for the technical aspects of its instrument to function correctly. "CHF put a lot of effort into making sure that the customer experience is complete," says LaTorraca. "The simple things, like the cart, have to be perfect on Day One. A panicky nurse has to be able to sprint down the hall with it."
A move from corded to cordless technology may also seem like a simple thing. In the case of the Avalon CTS cordless fetal transducer system, however, it has a big impact. With help from W. L. Gore & Associates (Putzbrunn/Munchen, Germany), Philips Medical Systems (Boeblingen, Germany) was able to give women in labor new freedom to move about, while maintaining doctors' ability to continuously monitor their babies.
Squeezing in technology
|
The Indigo Optima laser system from Ethicon Endo-Surgery Inc. |
In many cases, both high- and low-tech features have to fit into smaller and smaller spaces. "Technology is growing, but the operating room is stillthe same size," says Morelli. His team achieved a 35% reduction in the footprint of the Cardiac CryoAblation catheter and CryoConsole, whichis used by electrophysiologists to treat cardiac arrhythmias. The unit now integrates seamlessly into the cardiac cath lab, causing less interference with staff movement.
The endoscopic full-thickness Plicator from NDO Surgical (Mansfield, MA) also squeezes more technology into less space, consolidating the five separate tools needed for laparoscopic surgery into one instrument. It turns treatment of gastroesophageal reflux disease from a surgery requiring a two-day hospital stay into a 20-minute procedure requiring no incisions.
"Two key trends in the medical world are presenting incredible opportunities for advanced medical technology: ever-mounting pressure to hold medical costs in check and the need to reduce the possibility of medical errors," says David Robson of Item New Product Development (Providence, RI), which helped design the device. "Devices that enable noninvasive surgery and shorter hospital stays tap this trend."
The Indigo Optima laser system from Ethicon Endo-Surgery Inc. (Cincinnati) uses a fiber to allow minimally invasive treatment of benign prostatic hyperplasia. Because the device also shortens treatment time, local anesthesia is an option. Designers also made the device multifunctional--allowing treatment of bladder neck contractures, urethral strictures, and other conditions--to make it a flexible addition to doctors' offices.
While it doesn't compress technology, the CT Exprés does shrink both time and cost in computed tomography procedures that make use of contrast media. The unit's disposable elements and intuitive, modular design save clinicians time in training, instrument cleaning, and patient prep.
A different end-user
When the customer is not a clinician but a patient, OEMs face a new set of challenges that design partners can help them overcome. Guidant Corp. (Indianapolis) was in such a situation with its Partner Rhythm Assistant, and turned to Worrell Inc. (Eden Prairie, MN) for design assistance.
Richard Stein, principal system design engineer for product development at Guidant, says that the team from Worrell helped Guidant engineers spend time with patients, potential patients, and clinicians, allowing them to identify key needs for the Partner to fill. The handheld wireless device communicates with an implanted defibrillator and allows patients to check their own cardiac rhythm, and self-administer an atrial shock if needed.
|
The bion microstimulator from Advanced Bionics Corp. |
"The patients wanted the device to tell them what to do, and in their own language," says Stein, adding that Worrell helped the Guidant design team overcome their initial resistance to including voice technology in the product. The feature became what Worrell president and founder Bob Worrell calls a "patient delight."
Products designed for patient use also face new financial challenges. In the case of the Partner, costs had to be kept low because patients and their insurance companies were footing the bill. On the plus side, Worrell believes concerns about the cost of healthcare are leading OEMs to create more self-diagnostic tools like the Partner.
In some cases, however, financial pressures could squeeze truly innovative treatments out of the picture. Gerald E. Loeb, MD, director of the medical device and development facility at the University of Southern California's A.E. Mann Institute for Biomedical Engineering (Los Angeles), developed the bion microstimulator for Advanced Bionics Corp. (Valencia, CA). Reducing the size of the implantable neurostimulator potentially opens this treatment option to a wider patient population. "Pressures on healthcare costs will lead inexorably to an even more conservative environment for reimbursement of new treatments," says Loeb. "On the positive side, that could lead to more-objective, outcome-based decisions. On the negative, it could discourage many truly innovative treatments just because they don't already have a billing code."
The buck stops here
No matter what a product's purpose or prospective end-user, quality supply and design partners always keep cost in mind. "The design and development process associated with the creation of innovative new products must never lose sight of the manufacturer's primary goal--profit," says Herbst LaZar Bell's Keane. Even so, HLB is not generally a low bidder. "The differentiating factor in HLB's favor is an innovation process that generates intellectual property protection for many of the products developed for our clients," Keane says.
Item's Robson says he generally warns clients to beware of the lowest-cost bids. "These inevitably underestimate the complexity of a project. We try to identify and address all financial issues up front, so money issues don't derail the project before completion," he explains. "When money is taken out of the equation by establishing a realistic budget at the outset, it's much easier to tear down the traditional client/consultant barrier and take a fruitful team-based approach."
Robson sees new manufacturing technologies as a cost-cutting aid. "Technology innovation is enabling seamless communication between a manufacturer and supplier with truly collaborative software, Web-based phone conferencing, and Web-based PDM and PLM systems," Robson says. "Rapid prototyping methods have also changed a lot in the past five to six years, and will continue to become more accessible and cost-effective."
Looking offshore
With financial pressures, supply and design firms are also feeling pressure from competition. Halkey-Roberts (St. Petersburg, FL), supplier of the check valve for the System 100, stays in the game by developing new products with strong patents. "We continue to harvest our niche to stay on top of the market," says sales manager Steve Bello. A research and development partnership with a local university, as well as current projects, bring inspiration. "We're constantly looking to develop new valves," Bello says. "A lot of the ideas we get come from our customers. They have the experience with the end-user."
In the optical manufacturing sector, Elcan Optical Technologies (Midland, ON, Canada), supplier for the Indigo Optima laser system, has countered low-cost competition from Asian firms with new automated manufacturing and test equipment. "We have also invested in novel manufacturing techniques to investigate new technical capabilities, such as the production of nonspherical lens surfaces and the development of demanding thin-film coatings for optical beam splitters and filters," says program management director Evan Cameron.
Electronic manufacturing service providers also face a balancing act. "Firms are continuing to reconfigure their footprints to provide high-quality domestic services while offering low-cost offshore solutions," says engineering manager Corey J. Gannon of Smtek (Marlborough, MA), which worked on the System 100.
LRE Technology Partner (Nordlingen, Germany), a collaborator on the CT Exprés, stays on top of the market with investment and its excellent record. "We are in a good financial situation and can afford investments in new technologies and/or equipment to be competitive," says Ulrich Shroeder, CEO. "Another important point is our excellent quality management system and our quality record over the last 35 years in this specific business segment. We know what we are talking about, and this gives us a natural advantage compared to other companies in the EMS business."
B. Braun's Black says only companies developing new products that save time and provide what the end-user is looking for will be able totake advantage of the opportunities that the market brings. "As for the existing 'commodity' products, manufacturers are going to have to find ways to keep their costs low without compromising quality."
Consolidation will likely become an upcoming trend in the contract manufacturing industry, predicts Rose, with fewer but more-substantial companies offering more full-service options. Preferring to concentrate resources on their core competencies, OEMs will turn to full-service outsourcing specialists to handle the rest.
No matter how medical products and the companies that create them may change, however, certain factors will still be essential to the type ofsuccessful partnership that creates award winners. Cecilia Björkman of W. L. Gore & Associates sums it up: "Constant and proactive collaboration between manufacturer and supplier. Open communication and committed associates. Mutual confidence in the individual capabilities. The pursuit of a common vision/goal."
|
The MDEA competition is the premier awards program for the medical technology community, recognizing the many people behind the scenes--the engineers, scientists, and designers--who are responsible for the ground-breaking innovations that are changing the face of healthcare.
The program is open worldwide to companies and individuals involved in the design, engineering, manufacturing, or distribution of finished medical devices or medical packaging.
The MDEA program is presented by Canon Communications, the publisher of MPMN and also MD&DI, the program's sponsoring publication. Corporate sponsors of the 2004 competition include Avail Medical Products, DuPont Medical Packaging, the Medtech Group, Putnam Plastics Corp., and Nusil.
A complete list of this year's winners, as well as more information about the MDEA program, is available on the Internet atwww.devicelink.com/expo/awards02/index.html.
Copyright ©2004 Medical Product Manufacturing News
You May Also Like