This year’s MDEA contest saw a strong showing from international entrants. Manufacturers in China and India, in particular, are finding novel ways to cut costs without affecting quality.
March 25, 2011
Medical device technology is flat. In his book The World is Flat, Thomas Friedman proposed that globalization has altered central economic principles and has “flattened” the global marketplace. In other words, the barriers to international trade have been evened out, resulting in a more-level playing field for global commerce. This trend has been visible for some time in the medical device industry. Despite international regulatory hurdles, many companies face increased pressure to use global supply chain techniques, move production offshore, and respond to the evolving international marketplace. According to a report released earlier this year by PricewaterhouseCoopers (PwC) that provides further evidence of this trend, the United States may be the current global leader of medical technology innovation, but it is steadily losing ground to countries such as China and India. Not surprisingly, the influence of globalization was also evident in this year’s Medical Design Excellence Awards (MDEA) program. Out of the 36 award-winning products, 12 were either from companies based overseas or were the result of an international partnership. Several of these entries drew the most acclaim from the event’s jurors.
Cutting Cost but not Quality
In January, the Economist ran an article titled “Life Should Be Cheap: How China and India Can Help Cut Western Medical Bills.” It described how those countries might eventually help reduce medical bills in the West by selling us inexpensive medical devices. A growing number of Chinese and Indian firms specialize in developing products that cost substantially less than similar ones produced in the United States, Canada, and Europe. In most cases, devices being produced in these countries are not simply inexpensive knock-offs. They are frequently just as effective as the expensive technologies found in the West.
|
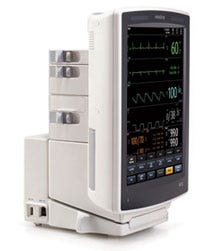
The sleekly designed V Series patient-monitoring system from Mindray costs about 50% less than comparable systems. |
Mindray, which is headquartered in Shenzhen, China, is a prominent example of a device firm that focuses on relentlessly reducing the cost of its products without sacrificing quality or efficacy. The company’s V Series patient-monitoring system wowed MDEA judges this year, owing to its immaculate design and impressively low price—it costs about 50% less than comparable systems. “The cost alone made it worthy of a nomination,” says MDEA juror John Sinacori, an assistant professor in the department of otolaryngology at Eastern Virginia Medical School (Norfolk, VA).
The industrial design of the device also caught the attention of the jurors. “Every little detail, is really well thought out,” says juror Bryce Rutter, founder and CEO of Metaphase Design Group (St. Louis). “The manufacturing and the precision of the tooling are flawless. That tool-set is just perfect,” he adds.
The manufacturer redesigned the user interface from the ground up to optimize it for a clinician’s workflow. The firm tested the unit in hospital environments, relying on end-users knowledgeable about the complexities of patient monitoring. Designed with flexibility and modularity in mind, the system can function as both a portable and bedside monitor. It can be undocked to facilitate quick transport. “It’s so flexible in where you can put it,” says juror Anne Miller, an assistant professor at the Vanderbilt University Medical Center (Nashville, TN). “The number of contexts in which you can use it is just really, really amazing.” The configurable parameter modules mounted at the back of the unit can rotate 180° to facilitate cable management. Juror Sean Hägen, founder of BlackHägen Design (Dunedin, FL), was especially impressed by how the unit provides a high amount of freedom with regard to cable placement. “The cables can come out from whichever side they need to come out,” he says. “That’s pretty innovative. Other systems out there force you to one side.”
The Vitality of Warmth
|
The Radiant Heat Warmer 3000 series was designed to redirect heat away from caregivesrs standing near the unit. |
Although China has become almost synonymous with low-cost manufacturing, India has given the country a run for its money in many ways. The Radiant Heat Warmer (RHW) 3000 series from Zeal Medical Pvt. Ltd. (Mumbai, India) provides an example of Indian ingenuity in cutting costs while preserving quality. Designed for the Indian marketplace, the open-bed radiant heat warmer for infants is 50% less expensive than other competing products in the market, according to the manufacturer. “Achieving that level of cost savings in a device like this is pretty significant,” Hägen says. “But the manufacturer didn’t sacrifice any important features by going there.”
Hägen believes that much of the unit’s costs savings stem from the manufacturer’s attention to design for manufacturing and assembly. “The tubing cross sections are all standardized sizes,” he says. “Construction methods also were standardized. It was very modular as far as the assembly is concerned,” he adds. “I thought just the innovation for design for manufacturing and assembly was sufficient for a nomination.”
The jurors also praised the clean form language of the unit’s design. “The design language is very consistent across all aspects: the hardware, the software, the design detail,” Rutter says. “There’s a nice rhythm and harmony when you look at it.” By contrast, many neonatal heating units look complicated. “Consider the neonatal intensive care units or any of the environments where you find these heating beds. When you think of it from the standpoint of the patient’s parents, all of that complication makes everyone a little bit nervous,” Rutter explains. “So we just thought it was nice that this unit could convey a kind of peaceful image back to the family.”
One of the principal innovations of the device is that it offers a unique reflector design that ensures uniform heating to the infant without excessively heating the surrounding environment. As the manufacturer points out, many radiant heat warmers are simply too warm to be around comfortably, limiting the time that doctors or other caretakers can spend at the cradle. To avoid this, Zeal Medical used a dual parabola radiant heater design that evenly distributes warmth across the entire cradle while redirecting heat away from the periphery of the unit.
Ensuring Safety and Stability
|
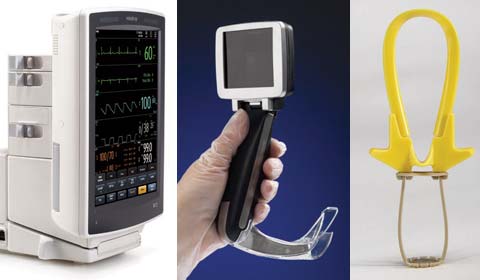
The Cranial Loop device enables a |
Cost reduction was a common theme addressed by most companies submitting entries this year, but so was ease of use, says juror Jay Goldberg, director of the healthcare technologies management program at Marquette University (Milwaukee). One product exemplifying this trend is the Cranial Loop from Neos Surgery S.L. (Donostia-San Sebastián, Spain), a plastic high-strength cranial fixation device that is used after a craniotomy has been performed. The device enables a standard cranial fixation procedure to be performed in less than one minute, according to the manufacturer. “When closing a craniotomy, fixating that piece of skull to the surrounding bone can be challenging,” explains juror Peter Denk, a surgeon based in Fort Myers, FL who specializes in minimally invasive surgery. “And this product has a really great, simple way of dealing with the issue.”
In a craniotomy, a section of the skull known as a bone flap is removed, giving surgeons access to the brain. The bone flap is normally replaced using metal plates and screws. This requires a number of tools to be used in the operating room and increases the risk that surgeons cut themselves during the procedure, explains Sinacori. By contrast, the Cranial Loop, which is made of PEEK-Optima, uses of a double-locking mechanism to close the bone flap. This design does not have any sharp points and can be locked in place without the use of tools. When closing the skull with the device, a single Cranial Loop unit is placed underneath each side where the cranium was cut. The handle of the device is pulled, tightening the loop portion, which compresses the upper part of the locking mechanism until a solid level of fixation is achieved. At this point, both sides of the loop are cut. The remaining strips are then bent back and forth until they break off at the base. This self-cutting functionality results in a low-profile finish that improves the safety of patients by minimizing the risk of soft-tissue damage.
A Practical Point of View
|
The A.P. Advance facilitates hand–eye coordination by giving physicians direct visual feedback of the patient's glottis. |
MDEA jurors also praised the A.P. Advance video laryngoscope from Venner Medical Singapore Pte. Ltd. (Singapore) for its ability to improve user experience. According to Industrial Design Consultancy Ltd. (Datchet, UK), the hand-held endoscopic instrument is said to make endotracheal intubation so easy to perform that a layperson can do it with some instruction. When designing the device, the design firm sought to integrate recent technological developments with a solid understanding of how laryngoscopes are used both in training and in clinical settings, says industrial designer Marko
Plevnik based at Industrial Design Consultancy Ltd.
The device features a 9-cm LCD view screen that positioned at the head of the unit, giving physicians direct visual feedback for eye-hand coordination. “The nice thing with this device design is that the screen placement enables you to always be focused on the patient,” Sinacori says. By contrast, some laryngoscopes have the video screen positioned to the side. “In the case of devices like those, when you are putting a laryngoscope in a patient’s mouth to intubate them, you have to turn your head,” he says. “That is a problem. When you are looking at that monitor and you’re trying to put the scope in, you’re taking your attention away from the patient’s mouth.” That lack of attention can increase the chance of running into problems, such as cracking teeth, during intubation.
The device is offered with a patented airway blade that has special tips that bend through a sharp angle. “The laryngoscope is designed to significantly reduce the learning curve for video laryngoscopy and provides high-quality video imaging of the airway in even the most-difficult clinical cases,” says Plevnik. A special guide plate directs the tube towards the glottis. The company reports that trials have shown that it is much faster when intubating difficult airways with its device than with other devices. “For me, it does seem to guide the tube better than some of the other intubation systems that are out there,” Sinacori says.
The device is substantially smaller than traditional laryngoscopes and was designed to optimize ergonomics. “One thing I liked about the design is how the plastic blades slip right on and they’re very easy to take off,” Sinacori says. He also was impressed with how the unit can be powered by a single AA battery. “You have to make sure that some laryngoscopes are plugged in or charged before use. There have been times when we’ve brought a laryngoscope into a room and it wasn’t charged. That can easily be a dangerous situation. With this device, it’s just a matter of popping in a new battery and you’re ready to go.” The viewer of the device also provides the option of using a rechargeable dedicated power supply.
A Vision of the Future
|
The Vedera KXS device could tap a new market for patients requiring mild-to-moderate vision correction. |
The aforementioned PwC report on medical technology innovation pointed out that U.S. consumers “are not always the first to benefit from medical technology and could eventually be last. Innovators already are going first to market in Europe and, by 2020, likely will move into emerging countries next.” This trend is reflected in the Vedera KXS vision correction device from Avedro (Waltham, MA), which could provide an alternative to Lasik for many patients with nearsightedness and keratoconus. Commercially available in several European countries and in Asia, the device underwent clinical trials in Europe and Turkey. The device is expected to be available in the United States in three or four years.
The company’s president and CEO David Muller, PhD explains that having an international focus has been absolutely crucial to his company’s business strategy. “A lot of domestic companies go outside of the United States with their products to help with their cashflow. For us, going into international markets has been a moving-forward business strategy. We wouldn’t be able to make money in the short term without doing that.” Muller helped popularize laser eye surgery in the 1980s by founding Summit Technology Inc., which introduced Lasik technology.
While treating mild- to moderate nearsightedness remains a potentially important market for the company, Muller is especially excited about the technology’s use to treat keratoconus—an otherwise untreatable disease that causes the cornea to deform. “We’ve gotten fantastic results using this technology to treat patients with keratoconus,” says Muller. “In some cases, we had patients who could barely see and, after the day after they were treated, they could go and drive a car.”
The device, which was designed by Continuum (West Newton, MA) and contract manufactured by Benchmark Electronics (Blanchardstown, Dublin, Ireland), uses microwave energy to offer a noninvasive, refractive correction procedure for reshaping the cornea. “The principle of operation behind this unit is that when you apply microwave energy to a region of the cornea, you heat it and change the protein structure to get tightening of the tissue, which then changes the cornea’s curvature to correct the patient’s vision,” says Goldberg.
This is similar to the basic principle behind Lasik surgery, but it differs in some important ways: First, it does not require
removal of tissue, as is the case in a Lasik procedure, during which a thin layer of the cornea is cut away. Second, the effect doesn’t have to be permanent. Theoretically, the system enables patients to try the procedure out. If they like it, they can be treated about a month after the initial procedure with a cross-linking procedure that makes the vision correction permanent. Otherwise, the effect fades in about three or four months, according to the companies clinical trials. “If, for some reason, the procedure didn’t work right, you could go and try something else,” Goldberg says. The cross-linking procedure is currently only approved for use in Hong Kong.
From the beginning, the company has planned on using the technology in conjunction with cross-linking, a new technology that is popular in Europe, according to Muller.
The jurors were also impressed by the industrial design of the device. “The ergonomics are good. The handpiece is nice, simple and easy to handle. I thought the design of the overall system was well done,” says juror Stuart Karten, founder of Stuart Karten Design (Marina Del Rey, CA). “It was apparent that this company really understands the vision correction market,” says juror Craig Jackson, PhD, the retired president of Hemosaga Diagnostics Corp. (San Diego). “I was struck by how the company approached manufacturability,” Goldberg says. “They construct the unit in two or three major components,” he explains. “When they ship it, it’s very easy to assemble at the other end. It’s designed to be very serviceable,” he adds. “And [the jurors] thought that, assuming this works the way it’s supposed to, it’s somewhat of a disruptive technology,” Goldberg says. “This could be a real competitor with Lasik.”
Muller acknowledges that the device might have a disruptive affect on the market, but he stresses that his firm really sees the device as additive technology. “Lasik has obviously been successful but when you look at the demographics, the people who have that procedure done overwhelming need a high level of correction,” he explains. “There is a huge, untapped market out there for people with mild or moderate myopia,” he adds. “There is a disconnect between the risk-reward ratio that Lasik provides. Our technology has the appearance of being much safer and less discomforting for that patient,” he adds. “We think our device is only disruptive in the extent that it increases the market.”
About the Author(s)
You May Also Like