July 7, 2001
Magnum Plastics to Produce Safety Syringe
|
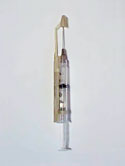
Magnum Plastics Inc. helped produce this safety syringe that reduces the risk of contamination for health professionals. |
A medical design company has selected Magnum Plastics System LLC (Erie, CO) to produce a one-handed safety syringe that lessens contamination risks for health professionals. Developed by DeHardCap Safety Syringe System LLC (New Orleans, LA; www.dehardcap.com), the syringe uses a spring-loaded plunger that is activated with a single finger. When the needle is removed from the patient, a plastic sheath with a stainless-steel hatch permanently caps it regardless of the remaining fluid level. A piggyback attachment, the product can be tooled for most existing syringes with minimal changes. According to DeHardCap vice president Lawrence Deharde, Magnum Plastics was selected for this project because of the firm's technical expertise and experience dealing with FDA. "Magnum," he explains, "had the necessary staff to work with us through the design and engineering phases all the way up to final production." Magnum Plastics, a contract molder, will manufacture and assemble the syringes at a new 20,000-sq-ft production facility. The FDA-registered company, which is certified to ISO 9001 and EN 46001, has 11 injection molding machines, an in-house tooling source, and a 2100-sq-ft cleanroom. Mass production of the syringe will begin in the fourth quarter of 2001.
Zachary Turke
You May Also Like