July 1, 2009
NEWS TRENDS
|
(click to enlarge) |
According to the International Trade Administration (ITA; Washington, DC), the world medical device market is expected to exceed $250 billion by 2012. As markets and opportunities develop, this number could increase, along with challenges. As a result, ITA is targeting four areas--quality, regulatory structure, pricing and reimbursement, and intellectual property (IP) issues--and is especially concentrating efforts in China, India, and Brazil.
As more manufacturers move overseas, quality will become a global issue, said Vince Suneja, director of the health product and technologies team in the Office of Health and Consumer Goods at ITA. Suneja discussed the global market at the Medical Device Manufacturers Association's annual meeting, held in Washington, DC, in June. One main risk, he said, is the introduction of low-quality products, which can weaken public confidence and negatively affect trade. ITA employs quality initiatives through training with industry and FDA on good manufacturing practices and counterfeit detection.
|
(click to enlarge) |
Differences in regulatory systems create trade barriers as well. In addition to encouraging transparency, fairness, and harmonization, ITA is working to ensure that devices aren't treated as pharmaceuticals. Other trade barriers include disparity in national reimbursement, which introduces adoption hurdles for more-complicated devices. ITA discourages such discrimination against imports and the use of foreign reference pricing, a method of cost containment.
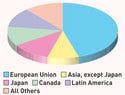
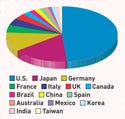
Although the U.S. market faces challenges, it remains a leader. It has held 45-47% of the global market in recent years. The largest recipient of its exports is the European Union at 46.4%, followed by Japan at 11.6%, according to the U.S. International Trade Commission.
Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like