The global market is looking up, according to a recent survey from the Emergo Group. The survey, “Medical Device Industry Outlook,” reflects a cautious optimism surrounding new markets, sales trajectories, and salary outlooks. The survey, in its third year, asked 1878 regulatory and quality assurance professionals about these trends.
January 27, 2011
Based on the responses, Emergo predicts a healthy rate of growth for 2011. For example, 74% of respondents said they expect sales to increase and nearly 70% of survey respondents had a general positive outlook for the medical device and IVD industry.
|
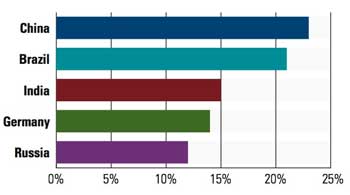
The top five international markets that survey respondents plan to enter for the first time in 2011. Note: respondents were able to choose more than one market, allowing for numbers greater than 100%. |
One interesting result of the survey is that it illuminated an outlook disparity between CEOs and their employees. CEOs were notably more optimistic about adding staff in 2011 (65.6%) than their employees (54.8%).
Chris Schorre, vice-president of global marketing at Emergo, posits that employees have been wearied by bad news about jobs and the recession. “The tenor of news has been negative with the economy and it may be that employees just feel beaten down.” He says that it bodes well, however, that CEOs, who are making decisions about raises and hiring, are optimistic about the next year. “CEOs may have a better view of the financials and are now beginning to think about growth.” Such an outlook is good news for everyone.
Speaking of growth, international sales will continue to be a driving trend, particularly into China, Brazil, and to a slightly lesser extent, India. Even though it is one of the BRIC nations, medical device firms continue to ignore Russia because of its complex regulatory structures, says Schorre. “Our clients don’t seem to have a tremendous interest because of its difficult regulatory process. It is very bureaucratic.”
China and Brazil, however, hold increasing allure, albeit with some challenges. The Chinese government has invested tens of billions of dollars building hospitals and clinics, which means it is a motivated market for medical equipment, Schorre says. “China is interested in reducing the inequities between cities and rural areas for healthcare,” he explains. Schorre says the Brazilian government is also investing to expand healthcare into rural markets.
One challenge with these emerging markets is that there are a limited number of documents in English. “The lack of documentation and knowledge of the regulatory process impedes these countries’ ability to attract outside investment,” Schorre notes.
Another challenge is that each market requires separate device registration, which means additional resources for firms looking to export medical devices. But respondents indicated that they understood the difficulties, saying that their most lucrative new markets are also the most difficult to enter.
The Emergo Group, in cooperation with the MedicalDevice Summit, conducted the survey in December 2010.
About the Author(s)
You May Also Like