Originally Published MDDI May 2006 News Trends
May 1, 2006
News Trends
|
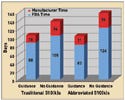
Figure 1. Average review times for traditional and abbreviated 510(k) applications in FY 2002 for Class II devices. |
FDA plans to create guidance documents as technologies are emerging—or at least in an earlier stage of product development. This approach would make such guidances available much sooner than in the past. FDA has noted a direct link between the approval time for 510(k)s and whether a guidance document was available for that product.
“A guidance is a way of helping get products to market sooner,” said Larry Kessler. Kessler, who is the director of the Office of Science and Technology at CDRH, spoke at the AAMI/ FDA conference in March. Guidance documents help industry understand FDA's expectations.
“If a guidance exists, explicit conformity with the guidance in an abbreviated 510(k) may further expedite clearance,” Kessler said (see Figure 1).
CDRH uses guidances extensively during reviews of both traditional and abbreviated 510(k)s, he said. Guidances are important, he explained, in light of the fact that a product can still be put on the market if it shows equivalency to a product approved in 1976. “It's not a pretty system, but we're working on it,” he said. “Guidances provide a road map for submissions and specify what CDRH believes will assist in the determination of safety and effectiveness,” he noted. “They level the playing field, but they also raise the bar of device safety, so we want to have them available for as many product types as possible,” he said.
To help get guidances produced more quickly, the agency is urging industry to submit its own guidance documents as well. “We are more than happy to receive guidance documents from [industry],” said Kessler. He noted, however, that once a guidance is submitted to FDA, it becomes an FDA document. “FDA has to make it its own, rewrite it according to plain language, send it out for comment for 90 days, and then issue it,” he explained.
Kessler noted that the globalization of standards and FDA's recent adoption of several large horizontal standards would also create “opportunities for economies.” For example, large horizontal standards such as ISO 13485 present opportunities for joint audits to standards, he said.
“Our vision within CDRH has been that standards are reactive. Standards have reflected the recent state of science,” he said. He emphasized that the agency wants to take an active approach to generate standards that will solve future problems. For example, he noted that FDA is looking at the safety and effectiveness of an artificial pancreas. “We are not waiting for the technology to be developed. We are working to develop standards while the technology is being developed,” he said.
Kessler stressed that standards and guidance documents can play a big role in getting products to market quicker. “As we develop standards, we need to be careful to be in areas that would not be restrictive. There has to be a balance so as not to put industry in a corner,” he said. “Guidance will be the CDRH focus.”
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like