Originally Published MDDI May 2004
May 1, 2004
Originally Published MDDI May 2004
Product Development Insight
Because it incorporates all functions and departments, a matrix management style is ideally suited for medical device projects.
David Kolesar and Frank Winterroth
|
David Kolesar |
Effective project management policies and practices are vital for successful new medical device product introductions. Effective policies are also critical in order to appropriately address compliance with U.S. requirements specified in the quality system regulation and international requirements in standards such as ISO 13485 and ISO 9001:2000.1–5 These principles apply to almost any business; however, they are of particular importance to medical device manufacturers because device projects are fairly complex and require expertise from multiple functional areas within an organization while complying with rigorous regulatory requirements to produce products that are safe and effective.
For medical devices, the project scope is usually relatively large and thus requires multiple communication channels across multiple functional areas. A company must also establish an internal quality system that ensures compliance with design controls and applies risk management principles. The personnel involved include corporate administrators, directors, and various department and group leaders associated with implementing and controlling the project.
|
Frank Winterroth |
With multifunctional project responsibilities, a traditional authoritarian (top-down) management style may not lend itself to the complexities of a successful medical device design and development program. In the top-down environment, the pressure is to conform to the directives from the top rather than to address the goals of the particular project. Therefore, a functional contributor may only be concerned with the issues surrounding the function's contribution and not with the success of the entire project.
A top-down management style may be an effective approach with simple designs and mass-produced products, but it lacks the commitment necessary for complex designs such as magnetic resonance imagers or positron emission tomography units. For such projects, the intricacy and complexity of the devices mandate that communication channels between the different departments involved (i.e., engineering, materials, manufacturing, regulatory affairs, quality assurance) be open at all times. Alienating any department or group leads to compromises in the integrity of the final product and increases project budgets and schedules.
The authoritarian approach often results in limited technical expertise within an assigned project team. In addition, a sense of divisiveness may emerge between individual project teams and the rest of the organization. These deficiencies can lead to missed milestones and cost overruns in the design and management of the project.
Matrix Versus Top-Down Management
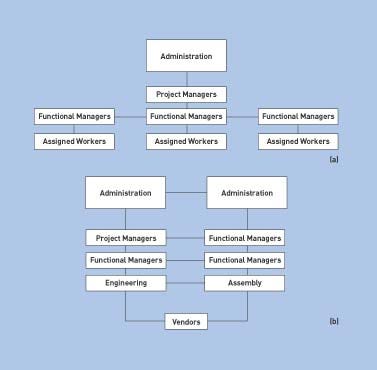
A matrix (or lateral) management style—in which the different functional areas are involved in the project through efficient collaborative channels (maintaining constant intercommunication between the different departments involved in the project)—is a more suitable approach. Unlike a traditional top-down management style, matrix management involves cross-functional interactions with authority and responsibility being shared among the participating departments (see Figures 1a and 1b for a comparison).
A key part of matrix management is the presence of team members empowered to make precise decisions with the ability to freeze the dialogue at ad hoc points during the project. In the top-down management approach, the administrative heads of either the company or the department oversee a project and hold the project managers accountable for its progress. Project managers then oversee functional managers in participating departments such as engineering, manufacturing, finance, and marketing. These functional managers in turn delegate responsibility to their assigned workers.
|
Figure 1. General comparison between (a) standard top-down management style and (b) matrix, interdepartmental style. Intercommunication channels between the various participants are maintained throughout the stages of a project when the matrix style is followed (Click to enlarge). |
In the matrix management example, administrators and managers still oversee and delegate responsibilities to their assigned department personnel. However, collaborative channels are also present so that each department and group involved in the project is fully aware of each other's assignments and progress. These channels provide a system for feedback among project members. This method is highly effective in keeping participants abreast of each phase of the project. Communication channels in the matrix approach maintain the functional top-down as well as the bottom-up channels, but add lateral communications across functional lines.
In the top-down management style, there is a high potential for miscommunication of the overall project scope. To minimize problems, communication channels must be constant throughout the course of the project. When the traditional top-down management style is applied to a typical medical device development organization (where juggling multiple projects is the norm), conflicts invariably arise over the relative priorities of projects in the competition for resources. A failure in any one channel could delay or even terminate a project. By contrast, the matrix style, which provides open communication channels and collaborative participation between all departments within a project's realm, enables greater input and feedback from all departments.
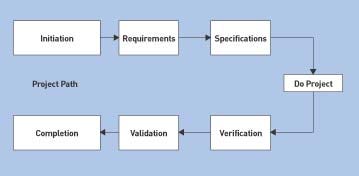
The matrix style can maximize the strengths and minimize the weaknesses of both project and functional structures because objectives are balanced through a project manager. Team members are more inclined to provide meaningful input to the overall project in a synergistic manner. Increased participation and cooperation leads to more variety and creativity. Ideally, project team members would work full time on a project, with managers and project administrators contributing to the planning process. The planning phase may range in length from a few weeks up to several months, depending on the scope of the project and the allocated schedule as specified in the project plan (which follows a standard project path schematic, as illustrated in Figure 2). The matrix style allows for maximum input into a project and provides multiple communication channels to ensure rapid resolution of conflicts.6
Collaborative production management (CPM) is the key to the proper functioning of the matrix management style and is essential to managing a medical device project (see Figure 1b).7 To successfully fulfill project criteria, the matrix management style incorporates and disseminates the project objectives to all departments involved. The elements of medical device design controls must be established within an organization regardless of which management style is incorporated.1-3
CPM lends itself to team building, open communication, and cross-functional project management. The project manager sees to it that the requirements established in the project plan are met. Project objectives include:
•Ensure correct translation of all design inputs into product specifications.
•Develop a worldwide regulatory strategy.
•Establish appropriate product design methods and procedures.
•Ensure that risk management is addressed at all phases of the device life cycle.8
•Demonstrate compliance with regulatory and industry standards.
•Specify challenges for various departments involved in executing different planning stages of the project.
•Produce a final design.
•Provide a clear understanding of the design transfer process requirements.9
•Allow for scheduling, technical quality, resource requirements, and balances.
•Ensure that manufacturing processes are developed, documented, and validated.
•Establish an approved vendor list, creating a vendor supply chain.
•Resolve cost-versus-quality issues.
•Overcome technical obstacles.
•Ensure appropriate implementation of design in manufacturing.
•Incorporate regulatory submission requirements.
•Follow policies, processes, and procedures established in the quality system.
|
Figure 2. General flowchart depicting the progression of any engineering project from project initiation through completion. Factors such as contingencies and risks are implemented as the project evolves (Click to enlarge). |
An effective project management style (in this case, the matrix format) is critical to planning, performing, and completing a project efficiently. The project manager has control over three factors: what has to be done, when it must be done, and how much in terms of resources must be dedicated to the project. Incorporating many objectives into such a format, however, can sometimes be difficult. As more departments, groups, and subgroups get involved in a single project, it is harder to properly organize and orchestrate their individual assignments. It also increases the likelihood of errors being incorporated into the project.
To define project responsibilities and deliverables, each department must regularly review the specific work instructions and operating procedures set forth by department managers and the project manager. The project manager is usually from engineering. However, the project manager may be chosen from another in-house discipline or hired from outside the company as an interim director. In either case, the manager's job is to lead the entire project team in planning the project—determining objectives, scheduling, and budgeting—and continually reviewing its status.
Periodic review of requirements ensures the overall effectiveness of the project's progress against the project plan and particular development phase. Review frequency depends on project time constraints, scale, and allocated budgetary contingencies. Major milestone reviews must be specified in the project plan. Requirements could include, but are not limited to:
•Sufficient resources for the project.
•Reasonable timelines, including initiation of project, and critical pathways (e.g., a Gantt chart to monitor milestones).
•Cross-functional (matrix) management involving all relevant departments: engineering, materials, manufacturing, business development, marketing, quality, etc.
•Communication channels between management, engineering, manufacturing, business areas, and other functional areas.
•A system or method to report project status to management with executive responsibilities.
•Appropriate design expertise.
•Feedback method for departments.
•A system to identify, address, and overcome obsolescence in production components.
Project Scope
Unfortunately, budget, time, and desired outcomes are rarely achieved as planned. If a project misses a schedule or a budget milestone, other deadlines and targets will likely be affected as the project progresses. Therefore, ongoing adjustments are necessary. Budget, time, and technical quality are all subject to ongoing review.
When identifying project requirements, it is important to keep in mind some guidelines that can help balance priorities within the project scope. Before funding a product design project, the design priorities must be studied, particularly in the case of computerized devices. Compromises between customer requirements and engineering capabilities are essential to achieving a project's objectives. At the start of a project, the following questions should be addressed:
•Does the device design follow the engineering pattern of Moore's law: does a tenfold increase in the product's memory and processing power become available every five years? (Moore's law was originated by Intel cofounder Gordon Moore and is used to prognosticate exponential growth in computing power. It has accurately predicted that the data density of computers would double approximately every 18 months.10)
•Is the company poised to meet this challenge, and what steps are currently being taken to incorporate new technology breakthroughs?
Furthermore, the project scope must address resources (including budget and time) that are necessary to achieve the desired outcomes. It is critical to review the scope of the project and determine whether any adjustments will be needed. It is also essential to identify which elements are essential and which are nonessential, to examine scheduling and resource constraints, and to factor in other appropriate resource issues as applicable.
In the matrix organization, each development phase has its own coordinating body that may only exist for that particular phase. Moving a project through the development phases is the responsibility of the project manager, with the project team fulfilling previously established deliverables for a particular phase as defined in the project plan. The project manager keeps executive management aware of the project's status. Executive management provides oversight for all new product development projects.
Tracking Project Progress
The matrix management paradigm requires developing and applying both a product breakdown structure and a work breakdown structure to a medical device development project.
|
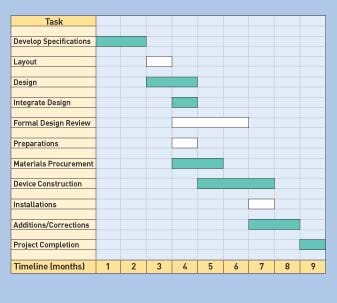
Figure 3. Example of a Gantt chart that illustrates the scheduling criteria for a medical device development project's phases. The critical path is denoted by a development the phases that are shaded. These steps are essential in order for the project to proceed to the next step (Click to enlarge). |
Breakdown Structures. The basic components of a work breakdown structure are work packages.11 A work package is an item in a system through which new medical device development can be tracked and successfully managed. By assigning individual tasks as work packages, management can track specific project expenses and progress against those tasks. When using CPM techniques, assigning and monitoring all work packages is essential to maintaining a team environment and ensuring functional contributions to a project's progress. A project manager should review each team member's work packages to determine whether the project's goals are being met and make adjustments as needed.6,11
The development of network diagrams and Gantt charts is an effective means of initiating, continuing, and completing a project in accordance with the general project path flowchart (see Figures 2 and 3).
Network Diagram. Developing a network diagram and a Gantt chart is useful for identifying a project's critical path and its critical chain. These elements can then be used to organize the project life cycle.
The network diagram organizes the work-package tasks and significant accomplishments (milestones) into a sequenced format that demonstrates how individual task dependencies fit together. The Gantt chart shows the project schedule. It describes the same events as the network diagram, but provides a time requirement for each task (see Figure 3).
Critical Path and Critical Chain. To determine the critical path, the project team must identify assignments necessary for progress, particularly tasks that are essential to the successful outcome of the project. All other project tasks are to be dependent on the critical tasks. Tasks on the critical path must be those that are necessary for the project to move forward. Establishing requirements and setting specifications must be the first two work packages on the critical path.
The critical path identified in the Gantt chart should represent the series of activities and tasks that must be completed in order for the project to progress as planned.12 Tasks with the longest time requirements on either the network diagram or the Gantt chart define the duration of the entire path. The sequence of activities is linked in succession and defines the overall project schedule (see Figure 3). These activities define the critical path for the project's success. Delays in these tasks will affect the project's progress and eventual completion targets.
Incorporating resource dependencies into the critical path produces a critical chain.12 The critical chain is the set of dependent tasks that defines the lower limit of the project's possible lead times. It also defines dependencies that are created when the completion of one task is required for another task to begin. In the critical chain, management reserves (i.e., contingencies such as additional time and budget) are incorporated. Emphasizing timeliness of task completion, the critical chain incorporates a buffer as a measure to control the project schedule. The critical chain also minimizes the need for team members to work on several tasks at the same time.
Comparing Control Methods
For medical device development, it is also useful to compare traditional and new-technology controls for project management.1,13 These controls are engineering design methods, technologies, and development processes applied within the medical device industry. Basic design control models are tested and have been used for years. If applicable, these controls are adapted by device manufacturers as benchmarks to their quality system requirements, allowing them to keep up with constantly changing markets.
Traditional. In the traditional approach, progress is measured via focus tracking against major project deliverables. In this approach, requirements change as new ones are added and as the schedule slips. Internal personnel are trained to address any new disciplines and specialties.
New Technology. When using the new-technology approach, project risks that could affect the schedule are tracked, and risk reduction is encouraged through safeguards such as concurrent development prototyping. Requirements are set so that they accommodate schedule targets. New requirements are evaluated to determine their effect on scheduling. This approach relies on recruiting from outside to address areas beyond the expertise of internal personnel.
Traditional controls correct risks and shortcomings as they arise throughout a project. New-technology methods control the design and implementation of a project from a big-picture context, exposing areas in which risks and pitfalls are possible. Strictly defined, new-technology design controls are project management skills and strategies that focus on quality and risk. These controls adapt readily to the continually evolving marketplace.
The new-technology approach lends itself well to incorporating design control requirements, such as quality planning for development activities, including verification, validation, quality, servicing, etc. The new-technology approach also places the design control requirements into the appropriate phase of the development process.
Conclusion
Project management principles must be applied in planning and executing successful medical device development projects. For medical devices, a project management style must incorporate specific regulatory requirements such as quality system requirements and design controls.
CPM can be an important tool in the successful design and implementation of a medical device project. To effectively implement CPM, communication channels must be developed that allow reporting from all departments on an ongoing basis.
A matrix management style sets up a system in which project managers oversee all levels of the design, development, and deployment of the project. It also enables project managers to report obstacles to management for review and correction. Because of its lateral structure, the matrix management style is ideally suited for product development of medical devices.
Acknowledgments
The authors would like to thank Robert Crockett, PhD, Lanny Griffin, PhD, and Daniel Walsh, PhD, of the Bioengineering Department at California Polytechnic State University (San Luis Obispo, CA) for their review and valuable insights for this paper.
References
1.Code of Federal Regulations, 21 CFR 820, “Quality System Regulation.”
2.P Palady, “An Emerging Core Competency for All Successful Managers and Engineers,” Regulatory Affairs Focus 7, no. 1 (2002): 29–33.
3.LR Pilot and DR Stone, “Legal Liabilities of Medical Device Outsourcing,” Medical Device & Diagnostic Industry 23, no. 4 (2001): 83–91.
4.ISO 13485:1996, “Quality Systems—Medical Devices—Particular Requirements for the Application of ISO 9001,” (Geneva: International Organization for Standardization, (ISO), 1996).
5.ISO 9001:2000, “Quality Management Systems—Requirements,” (Geneva: ISO, 2000).
6.GD Logan and DF Radcliffe, “Potential for Use of a House Quality Matrix Technique in Rehabilitation Engineering,” IEEE Transactions on Rehabilitation Engineering 5, no. 1 (1997): 106–115.
7.SA Vogel, “Satisfying QSR Requirements with Collaborative Production Management Systems,” Medical Device & Diagnostic Industry 23, no. 7 (2001): 90–96.
8.A Snow, “Integrating Risk Management into the Design and Development Process,” Medical Device & Diagnostic Industry 23, no. 3 (2001): 99–113.
9.T Zenner, “Preparing for Successful Design Transfer,” Medical Device & Diagnostic Industry 23, no. 8 (2001): 80–85.
10.MS Livstone, D van Noort, and LF Landweber, “Molecular Computing Revisited: A Moore's Law?” Trends in Biotechnology 21, no. 3 (2003): 98–101.
11.RK Wysoki, R Beck, and DB Crane, Effective Project Management, (New York: Wiley Computer Publishing, 2000).
12.LP Leach, “Critical Chain Project Management Improves Project Performance,” Project Management Journal 30, no. 2 (1999): 39–51.
13.D Oliver, “Developing Design Control Strategies to Meet Technology Advances,” Medical Device & Diagnostic Industry 22, no. 9 (2000): 77–85.
Copyright ©2004 Medical Device & Diagnostic Industry
You May Also Like