TECHNICAL PAPER SERIES
September 1, 1997
There is no standard protocol in the medical industry for using accelerated aging to determine the shelf life of packaging materials. Many organizations have developed test methods based on van't Hoff's principle, which describes the effect of temperature on the rate of chemical reactions.1 The application of this principle in determining accelerated-aging conditions for packaging systems is questionable, however, because of the complex kinetics involved in the accelerated aging of materials. Even as they search for a better test, however, the manufacturers of medical packaging are nevertheless responsible for somehow determining and describing the aging characteristics of their products.
To advance the quest for a reliable accelerated-aging standard for materials evaluation, DuPont Nonwovens (Wilmington, DE) recently conducted a survey to determine what conditions medical device manufacturers were using to evaluate accelerated aging. Based on the survey results, a set of accelerated-aging conditions was submitted to FDA as part of the company's Tyvek manufacturing transition protocol. These conditions--55°C and 7090% relative humidity, under which 14 days are assumed to be equivalent to 1 year of ambient real-time aging--were found acceptable by the agency, and are incorporated in the present research.
This study had three objectives. The first was to determine the effect of different sterilization methods on the adhesive properties of heat-seal-adhesive-coated, spunbonded HDPE (known throughout the industry by the DuPont trade name Tyvek). The second objective was to determine the effect of time on adhesive properties, regardless of whether the material was stored in roll/sheet form or as a finished, sterilized package. The third objective was to validate the accelerated-aging conditions by comparing results to real-time aging.
METHODOLOGY
A spunbonded HDPE heat-seal coated with an EVA-based waterborne adhesive was sealed to five bottom webs commonly used in the medical packaging industry. Three are materials employed as rigid thermoformed trays: PETG (18 mil, no silicone coating), PVC (18 mil), and HIPS (20 mil). The other two are flexible bottom webs: PET/PE (2.5 mil) is commonly used as the bottom web for pouches, and EVA/Surlyn/EVA (4 mil; denoted as E/S/E in Figures 13) is a formable bottom web used in form-fill-seal operations. The five different types of seals were subjected to ethylene-oxide (EtO) gas, gamma (cobalt 60) irradiation at 3.5 and 7.0 Mrd, electron-beam (E-beam) irradiation at 3.5 and 7.0 Mrd, and no sterilization.
Four adhesive properties were monitored: seal strength, transfer, cold flexibility, and sterilizer creep. Seal strength is the force (measured in g/in.) required to peel apart the seal, and was measured on a Thwing-Albert tensile tester using a 180° peel angle with a backing plate at a rate of 12 in./min. The spunbonded HDPE was pulled for the rigid bottom-web configurations, and the film was pulled for the flexible bottom webs. Reported strength values are for average seal strength. Transfer refers to a visual indication of the continuity of the seal; it was evaluated by rating the uniformity and brightness of the adhesive on a scale of 1 to 5, with 5 being brightest (a rating of 4 is typically considered acceptable in the industry). Cold flexibility--the ability to maintain seal integrity at low temperatures--was determined by flexing seals that had been exposed to a temperature of 10°F for 1 hour and measuring the percentage of seal that remained intact. Sterilizer creep is the ability of the seal to withstand high temperatures and high humidity during EtO sterilization. This was evaluated by measuring the degree of seal failure following exposure to a 25-g/in. stress for 40 minutes at a temperature of 150°F and relative humidity of 100%.
The four properties were evaluated before sterilization, immediately after sterilization, and after aging. As related above, accelerated aging was conducted at 55°C and 7090% relative humidity, with 14 days equivalent to 1 year of ambient aging. Real-time conditions were controlled at a temperature of 30°C and relative humidity of 5565%.
RESULTS
Effect of Sterilization. Seal strength, transfer, and cold flexibility were stable after exposure to EtO, gamma, and E-beam sterilization, with no detrimental impact on adhesive properties observed. In some cases, particularly with respect to seal strength and transfer, adhesive performance improved after sterilization. Figure 1 illustrates the seal-strength stability after exposure to each of the sterilization methods.
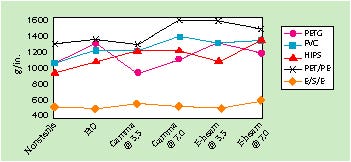
Figure 1. Effect of sterilization on seal strength.
Effect of Time Using Accelerated-Aging Conditions. Seal strength to PETG, PVC, HIPS, and EVA/Surlyn/EVA was stable after exposure to 10 weeks of accelerated aging (equivalent to 5 years of ambient aging). Statistical analysis does reveal significant differences in seal strength after certain aging periods on certain bottom webs. However, this analysis does not translate into poor seal-strength stability, since the differences either are not representative of a decline in seal strength, are not considered practically significant, or are not evidence of a consistent deterioration in seal strength over time. Seal strength to PET/PE was stable only for nonsterile samples (those that can be stored in roll or sheet form). After exposure to any of the sterilization methods, however, seal strength to PET/PE declined significantly with time. This is not necessarily attributable to a degradation of the adhesive system, but more likely is a result of a breakdown (delamination) of the film itself. Figure 2 illustrates the stability of this adhesive system when sealed to PETG (the most commonly used polymer for rigid tray applications).
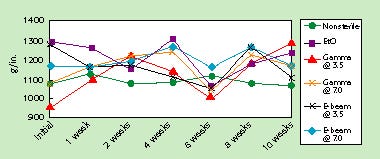
Figure 2. Effect of accelerated aging on seal strength to PETG.
Sterilizer creep was stable for up to 8 weeks of accelerated aging (4 years ambient). During this period, creep-resistance properties actually appeared to improve with time. After 10 weeks, however, sterilizer creep resistance tended to worsen, resulting in more seal failure. Because 10 weeks was the last test date evaluated, it is uncertain whether the results would constitute a consistent declining trend in adhesive performance over time. Results of the sterilizer creep stability testing are summarized in Figure 3.
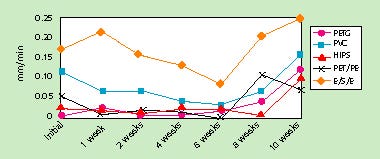
Figure 3. Effect of accelerated aging on sterilizer creep.
Transfer to PETG, PVC, HIPS, PET/PE, and EVA/ Surlyn/EVA was stable after 10 weeks of accelerated aging (5 years ambient). As with sterilizer creep, there appears to be an indication of a slight decline in transfer properties at 10 weeks, although the values obtained are still considered acceptable in the industry. Again, it is not certain if this represents the beginning of a consistent trend, as the 10-week values were the last data points gathered. Analysis reveals some statistically significant differences in transfer properties to certain webs after certain aging periods; however, as with seal strength, these are not practically significant. For the most part, transfer ratings throughout the study ranged from a 4 to a 5. The difference between these two ratings is minimal, with a 5 representing 100% bright white uniform transfer and a 4 representing near-100% (96+%) transfer that is somewhat less bright. Figure 4 shows the stability of transfer properties when the spunbonded HDPE is sealed to PETG.
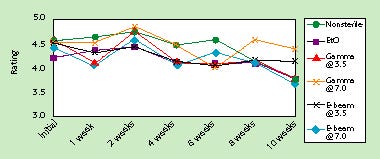
Figure 4. Effect of accelerated aging on transfer of seals to PETG.
Cold flexibility was stable after 10 weeks of accelerated aging (5 years ambient). As with transfer, although there were some statistically significant differences observed, these were not practically significant. Figure 5 illustrates the stability of low-temperature flexibility properties when the HDPE material is sealed to PETG.
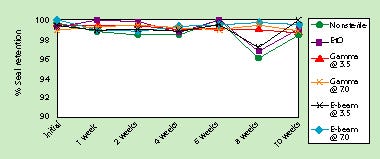
Figure 5. Effect of accelerated aging on cold flexibility of seals to PETG.
Effect of Time Using Real-Time Aging Conditions. Data has been collected after periods of 6 months and 12 months. All adhesive properties are stable. As with the accelerated-aging evaluation, statistical analysis reveals significant differences in some of the properties. Once again, this analysis does not indicate lack of stability: the differences either do not represent a decline in performance, are not practically significant, or do not indicate a consistent deterioration in adhesive performance to date.
Validation of Accelerated Aging Conditions. As yet, an insufficient amount of data has been generated regarding the effects of real-time aging. Therefore, it is not possible to validate the effectiveness of the accelerated-aging conditions. Data generated after 6 months and 12 months of real-time aging correlate well with data generated after 1 and 2 weeks of accelerated aging. Adhesive properties are similar or better after 6 months of real-time aging as compared with 1 week of accelerated aging.
Real-time aging effects on adhesive properties will continue to be evaluated. Once data have been compiled, an analysis will be conducted to validate the accelerated-aging conditions. We will conclude that an acceptable relationship between accelerated and real-time aging conditions exists if accelerated aging yields a similar or greater decline in adhesive performance.
CONCLUSION
The adhesive properties of the particular heat-seal-adhesive-coated, spunbonded HDPE (Tyvek) examined in this study remain stable after exposure to various sterilization methods and after undergoing up to 10 weeks of accelerated aging (assumed to be equivalent to 5 years of real-time aging). No practically significant decline in seal strength, transfer, sterilizer creep, or cold flexibility was observed, regardless of the bottom web to which the coating was sealed or of the sterilization method employed. To date, real-time aging results have been collected for only two test intervals (6 months and 12 months)--insufficient time to establish a relationship to the accelerated-aging data.
REFERENCES
1. Atkins PW, Physical Chemistry (3rd ed), New York, W.H. Freeman, pp 221223, 1986.
Erin Jones is materials product manager at Perfecseal Inc. (Philadelphia, PA), where she is responsible for all paper and Tyvek products used in packaging medical devices. Her previous position with the company was as an adhesive scientist in the R&D group developing heat-seal adhesive coatings for medical packaging applications. She holds a BS in chemistry from the College of William and Mary.
Copyright ©1997 Medical Plastics and Biomaterials
About the Author(s)
You May Also Like


