Award-Winning Devices Conquer a Variety of Challenges
May 6, 2006
Originally Published MPMN May 2006
SPECIAL FEATURE
From Drawing Board to Design Excellence
Award-Winning Devices Conquer a Variety of Challenges
Christina Elston
|
It’s unusual for a device manufacturer to get a call from the family of a patient. But that’s what happened to Eric Bailey, president of NeuroLogica Corp. (Danvers, MA; www.neuro-logica.com/index.html) one Thursday in March.
On the line was a Georgia neurosurgeon whose 14-year-old son was in a drug-induced coma at Children’s Hospital of Atlanta, his lungs damaged, his blood being oxygenated by a machine. Doctors were uncertain whether the boy’s brain was damaged, and needed confirmation of brain function in order to qualify him for a lung transplant. But his condition made moving him to another room for a CT scan too dangerous.
The father hoped Bailey’s CereTom mobile computed tomography scanner—which NeuroLogica loaded on an Atlanta-bound truck that day—could offer hope. Bailey himself was there to see the device in action. “I can say it was quite an emotional thing,” he says, adding that the scan showed the boy’s brain was functioning well. “At least now he qualifies for a lung transplant,” says Bailey. “There’s a chance this kid could live.”
The Medical Design Excellence Awards (MDEA) program, organized by MPMN publisher Canon Communications llc, seeks to spotlight products like the CereTom. The program, in its 10th year, considers the user, patient, and business benefits; design and functionality; and contribution to the advancement of healthcare of a host of products. Those products that the program’s judges, experts from across the medical spectrum, found worthy of silver and gold awards had to overcome a host of challenges to achieve their final form.
Back-Saving Work
|
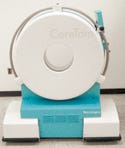
The world’s first portable CT scanner, CereTom, has circular handles in the vertical plane to provide many gripping places when moving horizontally. |
In creating the world’s first portable CT scanner, NeuroLogica also created a potential hazard for anyone moving the device around. The device is small but heavy, and industrial designer C. Michael Ault, president of Ault Design and Communications (Bedford, MA; www.aultdesign.com), didn’t want anyone trying to lift it.
“This was one dense gadget, and it had to be portable,” says Ault. “The engineers had sketched out horizontal handles at about the widest part of the cylinder. I worried that these would tempt the users to try picking the unit up while moving it.” The cylindrical form of the device had already been decided, so he made the handles echo those curved faces. “I came up with big circular handles in the vertical plane,” he says. “This discourages trying to lift the device, but gives lots of places to grip it when moving horizontally. They also serve as bumpers to protect the painted shells and allow the control panel to mount in different positions.”
Simple Access to a Complex Device
But NeuroLogica wanted more than portability. “NeuroLogica had some very clear ideas about what it wanted the user experience to be,” says Janice Archer, integration manager at Barco (Kortrikj, Belgium; www.barco.com). “It needed to be very uncluttered and simple to use, so that a wide range of people could operate the scanner.” The advanced visualization software provided by Barco for the scanner and its Clarus workstation made that possible.
Barco’s 3-D application, called Voxar 3D ActiveX control, allows customers to design their own user interface, and the company offers a custom-solution service to assist in this process. NeuroLogica also asked for new functionality to be built into the software. It wanted the device to be able to perform CT perfusion scans, and the Barco team had to identify and implement the correct algorithms. “That was exciting for us, to go into a new area,” says John Field, senior director of engineering and custom solutions.
Talking to Patients
In a home-use device, patients know what they want and manufacturers need to find out. To gather patient input and brainstorm concepts for its Cleo 90 insulin infusion set, Smiths Medical MD Inc. (St. Paul, MN; www.smiths medical.com) sought help from Bridge Design (San Francisco, CA; www. bridgedesign.com). The Bridge team sought out users and potential users—both young and old—of the type of portable insulin pump Smiths had in mind.
“We met some great people, and their desire to make things better for other people with diabetes really inspired us in working on this product,” says project manager and senior designer Diana Greenberg. “So many things really stood out that would be big improvements to this product.”
The collaborative effort between Smiths and Bridge proceeded with several brainstorming sessions on how to address issues like needle anxiety. The finished device keeps the needle invisible and provides semiautomated insertion, but still allows the user to control insertion speed.
To reduce the number of parts users had to handle, and the number of steps they had to go through, the design integrates the insertion tool—a separate piece in most other infusion sets—into the device itself. “You just twist off a cap and the set is ready to insert,” Greenberg explains.
A Sticky Situation
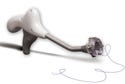
|
The Cleo 90 infusion set keeps the needle invisible and provides semiautomated insertion, but still allows the user to control its speed. |
Patients also needed the Cleo 90 to stay in place comfortably—a challenge Smiths presented to the engineers at Precision Gasket Co. (Edina, MN; www. precisiongasket.com). Senior sales engineer Duke Schneider, director of manufacturing Daniel Harvey, and senior manufacturing technician Walt Mengs worked two years to develop the manufacturing processes for the adhesive-coated artificial skin that secures the device comfortably on the body.
“No one will easily duplicate this product,” says Schneider. The size and proximity of the cuts to be made, along with the fact that different depths are required for each, meant the product required a very difficult tooling design. Multiple layers of adhesives had to be cut to varying depths within one-thousandth of an inch—simultaneously from top and bottom—on an unsupported skin with no structural integrity. “Then, on top of that, the part is very small and the wall thickness is very thin,” Schneider adds. “It was a lot of trial-and-error and tooling revisions and tooling designs. It’s a tricky little part.”
Essential Handles
|
Both large and small hands can operate the 360° Fascia closure device, a reusable mechanical suturing instrument that enables surgeons to close wounds. |
Sometimes getting the right handle on a device can make all the difference. This was the case for the 360° Fascia closure device, a reusable mechanical suturing device that enables surgeons to close wounds, manufactured by SuturTek Inc. (North Chelmsford, MA; www.suturtek.com).
The Bleck Design Group (North Chelmsford, MA; www.bleck designgroup.com) focused on the handle drive mechanism design, seeking a balance between creating leverage at the trigger and fitting into the smaller hands of female surgeons, according to president Jim Bleck. “Through engineering analysis, human factors analysis, and evolving models, we reached the current design,” he explains. “Both large and small hands can operate the device, and the sculpted shape assures users attain the proper leverage.”
Bleck says the design presented a chance to turn a limitation into a product feature. “The drive mechanism needed room to travel and caused the hump on the top of the handle,” he says. “We used this feature to create a saddle for the thumb and forefinger. Thus the hump became a functional feature, positioning the hand precisely for optimal control.”
The surprise while testing prototypes was the almost instant acceptance of the product by surgeons. Invariably, within seconds of first using the device, surgeons said, “This is really cool!” Bleck says, “The enthusiastic response was probably the biggest challenge during development because we had to channel the discussion to find the flaws.”
Making It Manufacturable
Concept design, of course, isn’t the whole battle, and SuturTek employed Lacey Manufacturing Inc. (Bridgeport, CT; www.laceys.com) to make the Fascia economical to manufacture. Lacey needed to develop, prototype, and verify the product design and provide initial clinical product within six months, followed by rapid cost-reduction implementation and production ramp-up, according to manager of manufacturing engineering Guy Osborne.
The team employed metal injection molding and investment casting to reduce the cost of the machined components. “The disposable needle and cartridge also presented significant technical challenges due to the dimensional precision and mechanical properties required to operate properly with the device,” Osborne says.
Late in the development cycle, supply problems requiring a materials change in the handles and trigger of the device threatened to upset the timeline. “Significant efforts were made in the rapid reverification of the design in the new material,” says Osborne. “Clear communication between Lacey and SuturTek related to the product development schedule, technical deliverables, and start-up production volumes were the foundation of this successful product launch.”
Molding in Micro
|
The Omni Pod insulin management system is made with a proprietary micromolding process. |
In the world of medical devices, “small” is “big” these days. And the OmniPod insulin management system from Insulet Corp. (Bedford, MA; www.myomnipod.com) is filled with plastic parts so small they are best described as “micro.” To keep the device, which delivers insulin at preprogrammed rates to people with diabetes, small and light enough for patients to wear, plastic component manufacturer Phillips Plastics (Hudson, WI; www.phillipsplastics.com) found itself faced with the challenge of “trying to get all of these small parts into a confined area,” according to business development manager Chriss Oseland.
Phillips uses a proprietary process called micromolding, which it has been working with for about three years, allowing it to produce plastic parts ranging in sizes from as small as 0.002 to 0.06 g, and metal parts that range from 0.013 to 0.375 g. One especially challenging part involved two-shot molding of a plateable-grade material and a nonplateable material. The plateable material was plated with a conductive metal designed to work with the circuit board of the device, and was used in multiple areas in the part. “It’s a very complex tool that produces that part,” Oseland says.
Moving Development Along
Before the OmniPod hardware was finished, the software and user interface for its handheld controller were already being developed and tested by Bay Computer Associates Inc. (Cranston, RI; www.baycomp.com).
By designing an application in Windows that simulated the display, and linking this to the code for the embedded system, Bay engineers could test and debug the software on a PC, according to Rick Sabourin, senior software engineer. This meant they could work on the content, layout, flow, wording, and icons, and even test them with users. As an added benefit, the application also provided a sales tool for Insulet Corp. to use in marketing the device.
“Medical hardware and software is always a challenge because there is such a high level of testing required, and so many safety concerns,” says Sabourin. Insulet seemed to understand the work that needed to be done up front to make the project a success, according to senior systems analyst Christina Vivona. “It was more open to a user-interface analysis than many new start-up companies are,” she says. “Sometimes it can be daunting to someone to look at the amount of work that has to be done up front.”
The Launch Point
|
The Inogen One oxygen concentrator system offers patients a portable traveling oxygen supply by concentrating oxygen from room air through a series of pressure chambers and valves. |
For the founders of Inogen Inc. (Goleta, CA; www.inogen.net), creators of the Inogen One oxygen concentrator system, their first challenge was finding a way to get their product vision off the ground. The device offers patients a portable, traveling oxygen supply by concentrating oxygen from room air through a series of pressure chambers and valves.
Help came from LaunchPoint LLC (Goleta, CA; www.launchpnt.com), a venture engineering firm that became a seed investor and technology partner for two years. “We collaborated on system models, digital and analog electronics, software, and mechanical design in preproduction prototypes,” says LaunchPoint president Brad Paden. Inogen, founded by three students from UC Santa Barbara, resided in LaunchPoint’s facility for those two years.
LaunchPoint’s mission, according to vice president of engineering Mike Ricci, is to provide engineering services to start-up companies, accepting stock in lieu of part of their regular consulting fee. “We specialize in understanding the array of technology that’s out there, and how to put those pieces together,” says Ricci. “We do what we’re best at, and spin it on to our client.”
Solving a Plumber’s Nightmare
Achieving the vision of the Inogen One also took design experience, so Inogen turned to Omnica Inc. (Irvine, CA; www.omnica.com). The company had previously brought portability to another type of oxygen device through its work on the Helios cryogenic system.
“Inogen came to us at a fairly early stage,” says Omnica president Rex Bare. “It had what I would describe as a breadboard.” The company had done some preliminary testing of its concepts, and Omnica worked with Inogen through the industrial design, prototyping, mechanical engineering, and packaging of the product, including design for manufacturability. It also designed production test fixtures for the product.
“Larger products in this category are sort of a plumber’s nightmare,” says Bare. Omnica developed a manifold assembly to contain the majority of the valves needed. “We built lots and lots of loose parts into a plastic manifold that had them basically molded in,” Bare says. The manifold provides paths between pressure chambers and forms the upper pressure head. This helps keep the weight of the device, and number of parts, to a minimum. “The labor went into the design, rather than into each unit that you build,” adds Bare.
Separating Oxygen
To address the adsorption-related challenges in the device, Inogen turned to Adsorption Research Inc. (Dublin, OH; www.adsorption.com). “The challenge was how to make a small, lightweight, and therefore portable pressure swing adsorption (PSA) system that could deliver enough oxygen at sufficient purity for a patient having COPD,” says president Kent Knaebel. “This meant keeping the adsorption part of the device small, and making it perform very well in order to meet the oxygen purity and quantity requirements.”
Though PSA has been used for more than 20 years to create home medical oxygen generators, “until recently, those devices were large and heavy, too much so for someone who suffers from chronic obstructive pulmonary disease (COPD) to move them, e.g., to travel overnight,” explains Knaebel.
No matter how rewarding it is for them to overcome technical challenges, though, most people involved in the creation of medical devices also think about the human impact of their products. Like Knaebel, whose mother suffered from COPD before her death in December. “Thanks to Inogen, she was able to have a portable oxygen concentrator,” he says. “It gave her freedom. Knowing that many other people can get that sort of freedom is very satisfying.”
Copyright ©2006 Medical Product Manufacturing News
You May Also Like