Medical Device & Diagnostic Industry Magazine MDDI Article Index
January 1, 2006
Medical Device & Diagnostic Industry Magazine Originally Published MDDI January 2006 NewsTrends
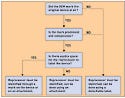
FDA has issued a draft guidance on how to implement a law requiring each reprocessed single-use device (SUD) to bear the name of its reprocessor. The Medical Device User Fee and Modernization Act of 2002 (MDUFMA) required any device to bear “prominently and conspicuously” the name, common abbreviation, or common symbol of its maker. The Medical Device User Fee Stabilization Act of 2005 amended that requirement to apply only to reprocessors of SUDs. No exemptions will be given. At the MDUFMA stakeholders' meeting in November, industry praised the guidance. Notably, reprocessors said they would have no problems complying with it. In many circumstances, the reprocessor's name will be put on a detachable label on the packaging, which could be affixed to a patient's medical record. Identifying marks can also be placed directly on the device or on an attachment to the device. The draft guidance defines prominent and conspicuous as: “A manner of marking a device, as required by section 502(u) of the [Federal Food, Drug, and Cosmetic] Act, such that the manufacturer's mark is apparent to the user under ordinary conditions of use.” The factors to be taken into account when determining whether something is “prominent and conspicuous” are available space on the device, contrast, meaning, and font or graphic readability. The guidance gives examples of things to think about when assessing each of those four factors. If a detachable label is used, it should contain a statement directing the user to remove it and put it on the patient's medical record when the reprocessed SUD is used. The draft guidance contains a flowchart designed to help reprocessors determine where to place their name or symbol (see Figure 1 above). CDRH wants the guidance to take effect by August 1, 2006. The full text can be found online at www.fda.gov/cdrh/comp/guidance/1217a.pdf. Copyright ©2006 Medical Device & Diagnostic Industry |
You May Also Like