The answer to how much money the agency spent on litigation in two crucial cases may be elusive.
September 1, 2008
WASHINGTON WRAP-UP
|
How much did FDA spend in litigating its cases against Utah Medical Products (Midvale, UT), which it lost in 2005, and TMJ Implants (Golden, CO), which is currently under appeal? Judging by FDA responses to two Freedom of Information Act requests I filed seeking revelatory documents, the agency does not know.
“This is to advise you that we have no responsive records from the Office of Chief Counsel,” wrote deputy chief counsel for program review Ann Wion in identical responses dated June 26.
In each case, my requests sought “legal expense summary documents showing accumulative dollar amounts and full-time equivalent (FTE) employee resources expended in all regulatory enforcement activities and [civil money penalties] litigation against [company]...”
FDA's response suggests that when it comes to initiating litigation, cost is not a consideration, a comment that provoked some lively reactions on my Web site, www.fdaweb.com.
One FDA employee indignantly said cost should never be a concern—public health and safety should be the only concern when FDA considers litigation. This, however, overlooks the fact that in the two cases for which I sought cost information, FDA did not allege that there were any public health and safety problems. Moreover, the question begs the issue of public accountability, of which keeping track of costs is surely a part.
Others suggested that since it is the Department of Justice that brings FDA cases to court, my cost inquiries should be directed there, instead.
So that is what I have done, as well as filing with CDRH for its investigational (as opposed to litigation) costs in bringing these two cases forward.
I'll keep you posted.
Schultz Defends Bench Test–Based Device Changes
|
Schultz refuted claims that FDA's policy is unsafe and said that Maisel's article “diverted |
Permitting medical device makers to modify an existing device based on bench testing is allowed by regulations and is not an unsafe approach, wrote CDRH director Daniel Schultz in a recent letter to the New England Journal of Medicine.
Schultz took issue with the March 6 article written by William Maisel that criticized FDA's handling of adverse events related to the recall of Medtronic's Sprint Fidelis implantable cardioverter-defibrillator leads. Maisel is the director of the Medical Device Safety Institute at the Beth Israel Deaconess Medical Center (Boston).
“In evaluating an application involving a modified device, we analyze the proposed modification, determine the potential types of failure, and tailor testing requirements accordingly,” Schultz wrote. “In many cases, our questions are best answered by performing appropriate engineering analyses, but in other cases, we also require clinical data…To require that these modified devices undergo clinical trials across the board as a condition of FDA approval would limit the availability of improved products. Also, most of these trials would have insufficient power to detect small but clinically meaningful differences in performance.”
Maisel's article criticized FDA and Medtronic over the Fidelis recall. After concerns arose about Fidelis's performance, he said, Medtronic notified doctors of a “limited number” of devices that had a higher-than-expected lead fracture rate. The company said Fidelis's performance was “in line with other Medtronic leads” and cited a small prospective postmarketing study that found a 1.1% lead failure rate within two years of implantation.
Maisel said the company didn't report that the study was “grossly underpowered to detect even a moderate increase in fracture rate in the Fidelis as compared with its predecessors. In short, despite implantation of the device in hundreds of thousands of patients during several years on the market, the available postmarketing data were insufficient to provide a definitive conclusion about whether there was a performance problem.”
Even as Medtronic maintained that the lead functioned within acceptable parameters, Maisel said, the company submitted an application to FDA in May 2007 for design and manufacturing changes and received approval two months later.
But already-manufactured leads remained available and continued to be used. By the following October, Medtronic had confirmed 665 fractures in returned leads, five deaths, and a 2.3% fracture rate within 30 months of implantation, and recalled the product.
Schultz's letter answered that there is “nothing inherently wrong” with allowing a device maker to continue to market existing models while modified (and presumably improved) models await FDA approval, unless the older models pose an undue health risk.
“The continued marketing of Fidelis leads occurred when available data indicated that the fracture rate was similar to that of other leads,” he said. “When continued monitoring of the situation showed otherwise, the lead was recalled and existing stocks were promptly called back.”
Schultz complained that Maisel's article “diverts attention from deeper problems. For example, how can bench testing be better designed to be predictive of clinical performance? How can postmarketing clinical registries be used more effectively as early warning systems, alerting us to low-frequency, unexpected problems with devices?” he asked.
Also, Schultz said, “Given that we cannot detect low-rate events without a steady flow of accurate information, how can physicians be persuaded to report adverse events to us promptly? We welcome thoughtful input on these issues from clinicians, patients, and the medical device industry.”
Advanced Bionics Settles U.S. Case for $1.1 Million
|
California-based hearing device manufacturer Advanced Bionics LLC (Sylmar) has reached a settlement with FDA. It will pay a civil money penalty of $1.1 million over alleged FDA reporting and adulteration violations.
The company failed to notify FDA of a change of outside supplier or vendor, which may have exposed patients to unnecessary health risks. Company president and co-CEO Jeffrey Greiner agreed to pay $75,000 under terms of the settlement.
An FDA news release said the company received FDA approval in July 2003 to market its HiRes90k implantable cochlear stimulator. But FDA alleged that Advanced Bionics shipped cochlear implants to U.S. customers without first filing appropriate supplemental information with the agency, including notice of a change of component supplier.
Advanced Bionics recalled the unimplanted devices with components from the unapproved supplier in 2006 because of excessive moisture, which could leak into the device and cause device failure and surgery that would have otherwise been unnecessary.
FDA's complaint also said the company shipped two devices containing the component from the unapproved supplier after the March 2006 recall, and the devices were subsequently implanted in patients.
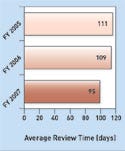
Device Reviews Improve, CDRH Says
|
(click to enlarge) |
CDRH's Office of Device Evaluation (ODE) received 9415 major submissions in FY 2006, up 8.3% from 8690 in the previous fiscal year. The ODE 2006–2007 annual report says the increase is primarily due to a jump in the number of premarket approval (PMA) supplements received. In FY 2007, the number received went down to 9276.
The average total elapsed time for original PMAs and panel-track PMA supplements has decreased overall from FY 2003 to FY 2006. The report says an increase in elapsed time in FY 2005 was likely due to a staffing shortage from that year because there was uncertainty over the continuation of the medical device user fee program.
The average ODE review time from receipt to final decision for 180-day PMA supplements has continued to improve, the report says. For the FY 2007 receipt cohort, the average ODE review time was 95 days, down from 109 days in FY 2006 and 111 days in FY 2005. The report also sees a “significant improvement” in the average total elapsed time for 180-day PMA supplements since FY 2005. For the FY 2007 receipt cohort the total time was 126 days, down from 169 days in the FY 2006 receipt cohort and from 238 days in FY 2005.
Encore Medical Cited over QSR Violations
Quality system violations were found in an inspection last January at Encore Medical's muscle stimulator and ultrasound device manufacturing facility in Hixson, TN, according to a June 25 warning letter issued by FDA's New Orleans district office.
Violations listed on the FDA-483 included failure to establish and maintain adequate procedures to identify actions needed to correct and prevent recurrence of nonconforming product and other quality problems.
The inspection also found that Encore failed or refused to furnish material or information required under the medical device reporting (MDR) regulations. FDA said the company failed to submit MDRs within 30 days of becoming aware that a marketed device may have malfunctioned and could have caused or contributed to a death or serious injury if the malfunction were to reoccur.
Encore failed to submit MDRs for multiple complaints concerning device malfunctions in which transient overvoltage within the circuitry caused components to fail. The malfunctions resulted in a shock, burn, or high output to patients, the warning letter said. Also, several reports were not completed correctly. Again, the warning letter said, the company's response was only partially adequate.
Encore was told to act promptly to correct the violations and was given 15 days to submit specific steps taken or planned.
FDA Seeks Comments on GHTF Documents
|
FDA is seeking comment on two medical device documents from the Global Harmonization Task Force (GHTF). GHTF's Study Group 1 has issued its final document, titled Role of Standards in the Assessment of Medical Devices. Study Group 5 has issued a working draft document titled Clinical Investigations. FDA wants to know whether stakeholders think the agency's regulations are inconsistent with any parts of the documents.
The standards document is one in a series describing a global regulatory model for medical devices. It describes the role of technical standards to demonstrate that a device conforms to essential safety and performance standards. This version supersedes an earlier guidance that has been changed to extend the scope to include in vitro diagnostic medical devices and provide guidance on use of recognized standards that have been revised or replaced. The guidance covers rationale, purpose, and scope; references; definitions; and general principles.
The clinical investigations draft is to provide guidance as to when a clinical investigation should be undertaken for a medical device to demonstrate compliance with the relevant essential principles. It also covers the general principles of clinical investigations involving medical devices. The guidance covers scope, references, definitions, and general principles when considering the need for a clinical investigation; general principles of clinical investigation design; and ethical considerations for clinical investigations.
CT Scans May Cause Electronic Medical Devices to Malfunction
|
CDRH has issued a preliminary public health notification on the possibility that x-rays used during computed tomography (CT) examinations may cause some implanted and external electronic medical devices to malfunction. The document has recommendations to reduce the potential risk.
FDA has received a small number of reports of adverse events in which CT scans may have interfered with electronic medical devices, including pacemakers, defibrillators, neurostimulators, and implanted or externally worn drug infusion pumps. And there have been similar reports in the literature. Reported adverse events have included unintended shocks or stimuli from neurostimulators, malfunctions of insulin infusion pumps, and transient changes in pacemaker output pulse rates.
The notification recommends that CT operators use CT scout views to determine whether implanted or externally worn electronic medical devices are present and, if so, their location relative to the programmed scan range. Other recommendations are given for instances in which a medical device is in or immediately adjacent to the programmed scan range.
Could Registry Help Thermal Ablation Trials?
FDA wants to know whether a registry could help standardize feasibility trials for local treatment of small breast cancers with different thermal ablation devices and therapies, according to a Federal Register notice. FDA particularly wants to understand how such trials can be designed to provide standardized evaluation of tissue
biopsy pathology, selection of tumors amenable to ablation, image guidance for ablation, postablation imaging and assessment, and tissue pathology of ablated specimens.
In 2003, the notice says, FDA's General and Plastic Surgery Devices Advisory Panel discussed issues pertaining to the use of thermal ablation devices to noninvasively treat breast cancer.
FDA says the panel's discussion has significantly affected regulation of the technologies. And investigators studying feasibility of thermal ablation devices for treating breast cancers have refined their techniques to the point that there have been small studies demonstrating nearly 100% ablation accuracy. However, FDA says, a lack of uniformity among different feasibility study protocols has yielded study results that can't be easily
compared.
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like