Technological advances in the cardio sector keep a pulse on patient needs.
July 1, 2008
COVER STORY: CARDIO TECHNOLOGIES
|
|
Illustration by GETTY IMAGES |
Underlying technologies are changing how patients interact with devices and how devices interact with patients' bodies. For the cardiovascular market, technologies such as coatings and wireless telemetry are helping healthcare practitioners serve patients better and more cost-effectively. New technologies are opening the door for more patients to receive stents. Wireless technologies are easing the burden on patients that have implantable cardioverter-defibrillators (ICDs) and pacemakers. It is impossible to touch on all of the advances that are affecting this segment of the device market, but a few of these exciting developments are detailed here.
The cardiovascular market is easily the biggest subsector within the device industry. Interventional cardiologists, particularly, tend to be early adopters and pioneers, says Leslie Bottorff, general partner, medical technology, of Onset Ventures (Menlo Park, CA). “Cardiologists are really aggressive in terms of wanting and demanding new treatments for their patients.
According to the American Heart Association (AHA), an estimated 80.7 million American adults (one in three) have at least one type of cardiovascular disease. Among these are nearly 1 million people with coronary artery disease—the focus of stent technology.
With such a huge patient population, it is not surprising that the stent market alone is very large. Bottorff puts the market value at more than $5 billion. Despite the fact that this segment has been hit hard over concerns about the late-stage clotting problems, it has managed to stay relatively strong as new technologies are developed to address those concerns.
The Stent Oligopoly
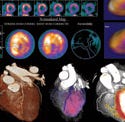
New scanning technology helps physicians detect cardiovascular disease. Click here to see the devices in action. |
Just a few years ago, the only drug-eluting stents available in the United States were Taxus from Boston Scientific and Cypher from Johnson & Johnson. The options are quickly growing: Medtronic just got its Endeavor stent approved and Abbott has recently released additional data on its Xience stent. Bottorff predicts that FDA will approve Abbott's stent by the end of this quarter.
“You had this game that had only two players and was beaten down for a bit. Now, these two companies are coming out with new stents with additional data, and the market is going to become a wider oligopoly,” says Bottorff. “They're all going to be competing for market share, and the shift is going to be what stent is right for what application and what patient.”
But Bottorff predicts that something else is going to happen, too. It is going to be a lot more important to have the best stent for a given subsector of patients—and that's where companies like CardioMind and Xtent come in, she says. Companies entering the stent market will have to compete for those patients that are best suited for its products.
CardioMind's Sparrow stent, for example, travels within the guidewire lumen to the site of the lesion. Xtent can customize the length and diameter of the stent while in the patient's artery. Such customization of stents may reduce the incidence of geographic miss, which has been associated with an increased risk of thrombosis and the need for reintervention. Bottorff says such unique characteristics are opening up the competition so that products are ideal for a given group of patients. “That's the trend you're going to see—a lot of these companies are still in clinical trials, but some of them are pretty far along. These characteristics can be leveraging.”
Big News for Small Vessels
|
|
|
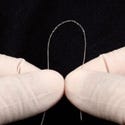
From top to bottom: Medtronic's new Vision 3D portfolio includes a full line of ICDs, CRT-Ds, pacemakers, and CRT-Ps; myocardial perfusion images in the Siemens's DynaCT show patchy hypoperfusion in the the left ventricle; CardioMind's Sparrow stent system. |
In coronary disease, the drug-eluting stents from a few large players are well known for handling most of the lesions that interventional cardiologists see. Among those companies are Boston Scientific with Taxus and Promise, Johnson & Johnson with Cypher, Abbott with its new stent, Xience, and Medtronic with Endeavor. However, in many cases, a cardiologist may choose not to use a drug-eluting stent if, for example, the patient has a very complex case and the doctor is worried about thrombosis. Doctors may also avoid using a stent if the patient may not adhere to the recommended dual anitplatelet therapy.
With a significant portion of patients going untreated, it's not surprising that quite a few companies are searching for ways to address this population. Those conditions have left the door open to the development of stents for special circumstances such as bifurcations, very long lesions, multiple placement of stents, or, in the case of CardioMind's stent, small vessels.
“The smaller companies that have pursued alternative platforms have looked at it as a $4.5 billion market when you add in the bare-metal stents,” says Charles Maroney, CEO of CardioMind. “When you talk to the interventionalists, the unmet needs or unsatisfactory outcomes are in those areas.” Maroney says that some of the large device companies have had programs to develop products for several of the branch or small vessels, but have not been successful in bringing a platform to the market. So, he sees this niche as an opportunity for smaller companies to enter the stent market.
The stent market got hit about a year ago when concerns about late thrombosis with the first-generation drug-eluting stents were reported. And interventional cardiologists began to question whether they should even put a stent in a patient if it might put the patient at risk.
The good news for stent developers, says Maroney, is that interventional cardiologists are driven by mechanical technical breakthroughs. “If you look at how the old balloon technology or the bare-metal stents have taken over markets and then, even now with the drug-eluting stents, you see the more-deliverable products capture more of the market. Interventional cardiologists are always in search of a better-performing product.”
|
(click to enlarge) Siemens's Syngo DynaCT Cardiac enables CT-like imaging of the heart. It eliminates the need for preprocedural CT or MR imaging. |
Maroney says that small vessels (from 2 to 2.75 mm) in the distal vascular tree present a perfect example because doctors have been dissatisfied with the deliverability of products in that area. This is not, he says, a job for a workhorse stent. “But, if this segment is 10% of the existing cases of a $5 billion market, it's very large,” he says. The profile of CardioMind's stent is 75% smaller than any other stent profile available.
The design of the CardioMind stent allows it to travel within the guidewire lumen to the site of the lesion. There, the cardiologist releases the stent and allows it to self-expand to the vessel wall. By contrast, conventional balloon-expandable stents travel over guidewires to the lesion, and thus, by their very design, occupy more volume, explains Maroney.
Because the Sparrow stent is more flexible than current stents, it is suitable for the treatment of the small, tortuous blood vessels often associated with diabetes. To coat the Sparrow stent, CardioMind has licensed the rights to the SynBiosys biodegradable polymer system from SurModics (Eden Prairie, MN).
“The value of our technology is that it's a self-expanding nitinol stent mounted on a guidewire,” says Maroney. “You use it just like you would use the guidewire in every case. It's 2–3 times more flexible than the stents mounted on the balloons. When we designed this system, we also integrated a biodegradable polymer so that in 6–9 months, the polymer is gone as well. Our struts, for example, are 65 µm thick. The next one up (one of the most deliverable) is the 87-µm thick Xience, which is expected to launch in the United States soon,” says Maroney.
Maroney says that if you've eliminated restenosis, and you have a gentle way of delivering the drug that produces no harm and reintegrates into the vascular tissue, that's an ideal situation. CardioMind is going after an even tougher challenge: a higher-value product for a lesion that requires a specialty stent. “Specialty stents tend to get a higher price than workhorse stents,” he says. “As time goes on, the specialty stents—whether they're for small vessels or bifurcations or long lesions or special stents for diabetic patients—those are going to be the ones that will command price premiums and command the attention of the cardiologists.”
Building on Biocompatibility
The idea behind MIV Therapeutics's stent technology was to find something that could ultimately deliver a drug to the vessels with a carrier that was either natural or biocompatible. “The most biocompatible substances are those that occur naturally in the human body,” says Mark Landy, MD. Landy is the company's president, CEO, and director.
Instead of a polymer coating, MIV uses a polymer-free material known as hydroxyapatite (HAp) to coat the metal and hold the drug, which is slowly released over time. Hydroxyapatite is a ceramic, similar to the mineral found in bones and teeth. For this reason, it is considered biocompatible and less likely than polymers to cause inflammation and infection.
|
(click to enlarge) |
“That was really the foresight and the understanding of how we got to this,” says Landy. “We've come up with a combination device, and we are able to make the hydroxyapatite porous. That porous latticework allows us to go into the well-proven lipid-based delivery systems. That's the rationale for going down the nonpolymeric route.”
There are drawbacks associated with nonpolymeric systems, says Landy. Such systems lack structural integrity, and they are typically unable to deliver the drug over the required time period. “We have proven that our drug-delivery system can deliver extremely hydrophobic drugs and very hydrophilic drugs,” he says. “As far as we know, we are the only company at this point that actually delivers drug capsules. When you deliver the drug in a capsule, you prevent the drug from washing out early if it's hydrophilic, and you help it from getting out of the coating if it's hydrophobic,” he says.
The Revolutionary Role of Imaging |
Ultimately, Landy says that if MIV's product continues to perform in large studies as it has in its initial human studies, the company will be setting a new bar for stents with respect to the combination of efficacy and safety.
“Drug-eluting stents have good efficacy; it's the safety profile we are worried about. Our ultimate dream is to have a stent that has drug-eluting stent efficacy with bare-metal stent safety. That is the gold standard, and we believe we are the closest to achieving that standard.”
Landy points to the issues that have contributed to the decline in drug-eluting stent usage, including a requirement for long-term Plavix therapy. “It's very well documented that the polymer contributes to the requirement for more drug,” he says. “Clearly we believe that removal of the polymer is a significant step.” Depending on the application, MIV's surface modification varies from 0.1 to 0.5 µm thick.
Landy envisions the MIV stent as a workhorse, noting that it is designed to be used in all patients, much like every stent is today. The difference, he says, is its flexibility. “We've been able to manufacture a drug-delivery system that is so small that we do not alter the underlying handling characteristics of the bare-metal stent.” Landy says the company would like to launch the stent in Europe within the next 24 months.
Polymer Applications: Cardiac Stents Evolve
Prevention: The First Step |
“Currently the majority of stents used in cardiac applications are metallic, which in many cases use a polymer coating for drug elution; however, there has been a migration toward complete polymer stent substrates over the last few years,” says Khristine Carroll, global director of sales for AdvanSource Biomaterials.
Stent designers, she says, are developing polymeric stents to replace the currently prominent metallic products, which can mimic natural arterial pulsatility in more-delicate arteries while maintaining radial force. She says this promotes polymers as one of the choice materials for new development initiatives.
“Historically, polymer coatings have been impregnated with pharmaceutical preparations in an effort to reduce the occurrence of restenosis and vessel blockage after stent placement,” says Andrew Reed, PhD, AdvanSource Biomaterials's vice president of science and technology.
Some current drug-loaded polymer coatings have suffered serious side effects in terms of reported elevated rates of late-stage thrombosis. Further investigation has shown that the thrombosis may be caused by the cracking and fragmentation of the drug-eluting stent coating.
Companies such as AdvanSource Biomaterials have developed biodurable, drug-eluting polyurethane coatings that are designed to adhere to both metallic and polymeric stents. Such coatings are immune to environmental stress cracking and metal ion oxidation.
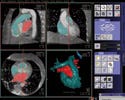
|
(click to enlarge) |
AdvanSource has also developed a family of antimicrobial polymers intended for use in the manufacture of catheters and other medical devices. Through proprietary manufacturing techniques, the company has produced materials that allow for homogeneous dispersion throughout the polymer, resulting in long-lasting and consistent activity. Other advances include radiopaque agents that support visibility of the device during the insertion without losing flexibility critical to the procedure.
“The coatings can be tailored to obtain specific drug-elution profiles that depend on the specific application. They are designed to release a wide variety of pharmaceutical agents at controlled rates throughout the life of the stent,” says Reed. “These materials have been molecularly engineered to provide mechanical properties that allow the coating to remain intact during the expansion of the stent during deployment.”
These second-generation, tailored polymer coatings allow delivery of pharmaceutically active agents in a controlled fashion with customization to various elution rates and time without the potential for cracking and fragmentation. “Such characteristics reduce the likelihood of late-stage thrombosis and restenosis,” says Reed.
It is the advances in polymer technology that are enabling more-elaborate device development. “Companies use polymers as the full structure in order to gain the elasticity and flexibility needed to overcome tortuous anatomies, while still maintaining critical characteristics such as device pushability and torque control,” says Carroll.
Of particular interest, she says, are stents manufactured from biodegradable and bioabsorbable polymers. For example, AdvanSource Biomaterials is developing a product family with controlled-degradation capabilities to address this trend.
“It has been shown through postmortem studies that plaque morphology and composition play a significant role in predicting events such as restenosis and stent thrombosis,” says Carroll. “Pharmaceutical companies are researching the possibility of developing unique drug-based therapies tailored to the specific plaque observed in a particular patient.”
The tailoring of drug-eluting stents to reflect individual patient morphology and plaque composition is in early-stage development. Carroll predicts that this will be a significant development that will allow cardiologists to provide designer treatment for individual patients.
“As with many invasive procedures, the placement of stents in the patient includes delivering the device through the vasculature using balloon catheter technology. Insertion of such devices exposes the patient to potential bacterial contamination and infection,” explains Mike Adams, CEO of AdvanSource. “To prevent bacterial infiltration and proliferation, antimicrobial polymers will be used to manufacture delivery catheters.”
Transcatheter Valves: Addressing an Unmet Need
Advances are not limited to stent development, however. Transcatheter valves are also seeing a lot of innovation. The great leap forward, of course, is that with these valves, surgeons no longer have to cut a patient's chest open to replace the defective valve with a new artificial valve. The new valve can now be delivered through a catheter.
“The area that's furthest along is the development of aortic valves,” says Bottorff. The companies in this sector are Edwards Lifesciences and CoreValve. Both companies have valves that have been released in Europe. The Edwards system is in a pivotal trial in the United States.
“The only pitfalls of the Edwards and CoreValve products is that once you release the device in the patient, if it's in the wrong spot, the patient will be going to surgery. You can't get it back again,” Bottorff says. “These are patients who are already at high risk for surgery.”
Bottorff notes that the development of these minimally invasive valves is a reflection of evolution in cardiology. Right now heart-valve replacement requires surgery in most cases. But, she says, there are many people who aren't even eligible for surgery.
“You'll see an evolution to offer something percutaneous to those people, and assuming that this option is successful—which it is looking to be at this point—there are a lot of surgeons and interventional cardiologists who think the use of a transcatheter valve will become the primary method used for anyone who needs an aortic valve.”
Edwards Lifesciences is also studying the use of the transcatheter valve to address a congenital condition in which the valve between the right ventricle and the pulmonary artery is nonfunctional. In April this year, the company announced that the first patients were being treated in a U.S. feasibility study using the Edwards Sapien transcatheter heart valve for pulmonic valve replacement. FDA conditionally approved the investigational device exemption clinical trial in late 2007. The study entails 30 patients at three hospitals.
The Edwards Sapien bovine pericardial valve is compressed onto a balloon to the approximate diameter of a pencil and is threaded through the patient's circulatory system from the leg using the RetroFlex transfemoral delivery system (for the aortic or pulmonic positions), or through a small incision between the ribs using the Ascendra transapical delivery system (for the aortic position). The valve replacement is accomplished as a beating-heart procedure, without requiring cardiopulmonary bypass or an open-chest incision. Because the minimally invasive procedure is less traumatic, it addresses a critical unmet patient need.
“Interventional cardiologists see this as the next wave in valve replacement,” says Bottorff. “It's the next thing they're going to be able to do for their patients minimally invasively. Enthusiasm for transcatheter valves continues to build.” Bottorff notes that steady progress is being made in Europe in terms of reimbursement for these valves, and that the National Institute for Health and Clinical Excellence in the UK recently issued a favorable provisional decision to reimburse the procedure.
The Wireless Revolution
|
(click to enlarge) |
Medical technology has begun to implement many technologies that people take for granted in many other aspects of their lives. For example, Medtronic is among the cardiac device companies taking advantage of advances in wireless technologies to improve its products. It has received FDA approval for the first wave of its wireless, remote monitoring cardiac rhythm disease management therapies, which includes a line of ICDs, cardiac resynchronization therapy-defibrillators, pacemakers, and cardiac resynchronization therapy-pacemakers.
Medtronic is blending its platforms for its ICDs and pacemakers, focusing on what it calls “important refinements” to its products. “Some of them will be important for physicians, some will be important for patients, and some will be important for both,” explains David Steinhaus, MD, vice president and medical director of the cardiac rhythm management division for Medtronic. Steinhaus says this strategy will enable the company to leverage the combined platform so that future developments will be quicker and less expensive. Even though he calls these changes “refinements,” Steinhaus says that there are a couple of advances that are game changing for the market. These products, he says, offer innovations that will significantly improve heart rhythm therapy for patients.
The first innovation, says Steinhaus, is the idea of complete capture management. Rhythm management devices have three different channels—an atrial channel, a right ventricular channel, and a left ventricular channel. The device can capture data from all three automatically. It will know the thresholds in each chamber and will be able to adjust the output for each chamber. Capturing data automatically improves battery life and helps physicians manage patients better, he says.
Steinhaus knows the value of these improvements firsthand because he is a cardiologist. “Before I came to Medtronic, I was based in Kansas City. Some of our patients who had this type of therapy lived in western Kansas. They had to come to the office every so often to get checked. That typically meant getting the son-in-law (because they live on a farm somewhere) to drive the parents six hours to Kansas City. I would see them for 10 minutes to check the device and then they would make the six-hour trip home. That's really unproductive.”
Complete capture management sets out to eliminate routine checks of the device. The capture-management function is combined with audible alerts that come with wireless technology in the company's CareLink products.
“When there is a problem, the device will tell the doctor, who can have the patient come into the office. So you can imagine what a tremendous boon this is to patient care. You don't need to see the patient unless there is a problem,” he says.
“Medtronic takes its role in the cardio technology market very seriously. We've done a lot of really cool things to do that. If you look over Medtronic's product line, you can see that the company has been marching in the wireless, remote-monitoring direction over the last 5–10 years,” says Steinhaus. The development of the CareLink network, which was launched in 2002, allows physicians to remotely access patients in their homes.
Wireless telemetry in these heart rhythm devices now takes the interaction of the patient out of the picture, so patient compliance is no longer a factor. Steinhaus says that the device can be placed at the patient's bedside and can check in while the patient is sleeping, where before the patient had to put the programmer head over the device.
“The devices automatically check themselves, check the thresholds, and set the thresholds. So if there's a problem, they automatically notify the physician's office. That allows the doctor to say, ‘All right Mr. Jones, go back to [your town], use this, and it will tell us if there are any problems. Otherwise, from the standpoint of your device, I don't need to see you until (or if) there's an issue or if the battery starts to wear down.'”
“The second game-changing deal,” says Steinhaus, “is that we're offering a full product line that has different features for different patients—all on the same platform. That's a really big deal. Complete capture management, for example, will be one feature that is not offered on all of the devices. Some patients need a basic device, and some need a much more complex device,” he says.
Medtronic is also taking advantage of advances in sensor technology. Its first sensor, called OptiVol, provides fluid status monitoring to measure intrathoracic impedance in heart failure patients. The cardiac giant is planning to add more physiologic sensors in these devices, too. “We're going to have other sensors that will send physiologic data automatically to the physician's office,” says Steinhaus.
Medtronic is planning to add a pressure sensor that measures pressures inside the right ventricle and that can help manage heart failure. Steinhaus says there are also plans for devices to have feedback control loops. So, the device can measure how the heart is doing and then either alter the way it paces or alter the way it functions based on that measurement. It will then change dynamically over the course of the lifetime of the patient and the lifetime of the device, perhaps even using drug pumps.
Steinhaus says that Medtronic is also concentrating on trying to minimize the number of shocks that patients get. A shock from an ICD can be somewhat painful, but it's lifesaving. Medtronic is managing the way this device gives therapy—calling it pain-free therapy. “Another advance that will evolve over the course of years will be leveraging what we've done with the CareLink,” he says. “With the wireless telemetry, and now with the automaticity, adding sensors to that will be pretty exciting.”
Addressing Congestive Heart Failure
|
The Sapien transcatheter heart valve from Edwards Lifesciences. |
Many people in the United States die from congestive heart failure, and it costs the U.S. healthcare system a ton of money. According to AHA, about every 26 seconds, an American will have a coronary event, and a person will die from one nearly every minute. AHA estimates the cost of cardiovascular disease at $448.5 billion for 2008. Everyone wants these patients to be served better from a morbidity and mortality standpoint and well as more cost-effectively. The medical device industry is certainly playing a huge role in addressing the problem.
“It's a disease with a lot of variables,” says Bottorff. “It takes a long time to get clinical trials done. There have been a number of companies funded in this area that ended up not being successful because the investment in the clinical trials is very large. Nevertheless, it's a big market opportunity, so there will be companies that are great investments in this area.”
One example, she says, is BioControl's CardioFit system, an electrical stimulator system designed to improve heart function. According to the company, the implanted neurostimulator includes a microcomputer that analyzes information from the heart and decides on the appropriate stimulation needed at the vagus nerve. This stimulation is carried through an electrode from the implanted neurostimulator to the vagus nerve in the neck. The company says the electrical stimulator system may be able to reduce the heart's rate, restore regular rhythm, and reduce inflammation.
Conclusion
Innovation is evident throughout the cardiovascular market. Whether it's the drive of the doctors or the needs of the patients, technology is pushing developments in stents, heart valves, and cardiac rhythm disease management, just to name a few. Advances in imaging and preventive devices are also playing a role in the growth of this market.
According to Applied Data Research (Amherst, NH), more than two dozen technology-driven companies are pursuing development-stage stents designed to provide improved patient outcomes by eliminating or at least mitigating the problems of marketed devices. In particular, efforts to correct problems in the current generation of coated devices has led to an intense level of activity. And the baby boomers are leaving their mark, too. They are a generation that expects more from medical technology. Wireless capabilities in devices are a huge step toward improving healthcare efficiency and patient quality of life.
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like