Medical Device & Diagnostic Industry Magazine MDDI Article Index
January 1, 2006
Medical Device & Diagnostic Industry Magazine Originally Published MDDI January 2006 NewsTrends Erik Swain
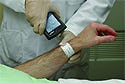
Concepts like six sigma and lean manufacturing are traditionally thought of as being used in internal processes. But why can't device makers apply them to end-user environments as well? Might that affect cost reduction and patient safety? That's what BD Diagnostics (Franklin Lakes, NJ) is hoping for with its latest specimen-collecting system. The BD.id system is designed to cut costs and improve patient safety. It will ensure that lab specimens are collected into the right container, labeled in the presence of the patient, and linked back to the correct patient. BD hopes to reach these goals by using the same techniques it employs in its own design and manufacturing processes. “One key thing that we asked was how could we take techniques like lean manufacturing and six sigma and drive them into the hospital environment,” says Beth DiLauri, senior marketing manager at BD Diagnostics. “If we could eliminate unnecessary steps by applying lean management, where you figure out what to cut out to save time and resources, wouldn't that also reduce the opportunity for error and be safer for the patient?” The process begins before the hospital staff even uses the system. “We ask how standardizing the process will affect this specific hospital,” says DiLauri. The BD consultants use six sigma, lean management, failure mode and effects analysis, and other techniques to analyze a hospital's current specimen collection process. This includes examination of the layout of supplies, time-and-motion studies to cut down on unnecessary movement, and a return-on-investment analysis to determine potential financial benefit. The system itself pairs an advanced software program with bedside bar coding technology. When a specimen collection order is issued, a server downloads it and sends it to a handheld computer with a bar code scanner. The collector scans his or her badge and the patient's bar coded wristband before taking the specimen. The handheld device prints a label listing the collector, the patient, the date and time of collection, the type of container used, and the type of test ordered. The collector then uploads that information to the server for the lab to download. Loading data immediately means lab staff can track specimens that haven't arrived yet and learn where the collectors are. And the entire turnaround time can be tracked. The latest version of the device is wireless and uses a Microsoft Windows 2005 operating system. “Everybody knows that ensuring patient safety is the right thing to do, but it's difficult for hospitals to find the resources to fund it,” says DiLauri. “We are working with hospitals to build the case for it. For example, one thing we look at is redraws. If you do a redraw, what does it cost in resources and time, including reanalysis and rediagnosis? And what can you save if you cut down on redraws by standardizing your procedures? Additional handling reduces the value of your system.” The system has reduced misidentifications to far below 1%, and it has even gotten it to zero in some hospitals, DiLauri says. The staff at Jackson-Madison County General Hospital in Jackson, TN, has found great value in the system. “We learned that we had a lot of wasted time and non-value-added steps in our process. We were not standardized in how each [collector] approached the collecting process. We were surprised at how many times specimen labels were handled and resorted,” says Jamie Boone, the hospital's assistant director of laboratory services, about the BD consultation. “We [now] see the BD.id system as a perfect fit for our hospital. It will allow us to identify patients, reduce the time wasted in continuous handling of labels, and reduce the errors associated with collecting in the wrong tube.” The next step, DiLauri says, is to apply the process to blood transfusions. Copyright ©2006 Medical Device & Diagnostic Industry |
About the Author(s)
You May Also Like