Originally Published MDDI February 2006An MD&DI roundtableDesign experts discuss the demands that come with more globalization, including how to develop products that serve multiple markets.Michael Wiklund
February 1, 2006
An MD&DI roundtable
|
The medical device industry is globalizing. As part of that trend, companies are developing products for sale worldwide rather than developing devices just for a particular market. This shift has placed new demands on design teams. The purpose of this roundtable was to identify these demands and to share opinions on how to best address them. The participants in the roundtable, Michael Wiklund, Torsten Gruchmann, and Stephanie Barnes, have backgrounds in human factors and user-interface design (see the sidebar, “Meet the Panel,” on page 69). With their expertise in mind, the discussion focuses specifically on the best or most practical way to develop user requirements.
Do you agree or disagree that an increasing proportion of medical devices are being developed for a global market?
Gruchmann: Most European companies are more interested than ever in exporting their products. They can tap a much larger market than is possible serving their domestic needs. There seems to be particularly strong interest in selling to Asia—China in particular.
Barnes: I agree that medical companies are designing an increasing proportion of products for global use. Approximately 50% of SonoSite's sales are international. It's a mind shift for a company that started off focused primarily on the U.S. market. SonoSite now has eight direct subsidiaries and is selling into 75 countries, and so we are reminded every day that we're working on international products.
|
Stephanie Barnes |
What is your company's strategy for developing user requirements for global products?
Barnes: We don't have a single process or group of people responsible for defining international product requirements per se. It tends to be more of a collaborative effort among people working and living in the international communities and those working back at headquarters.
How does developing requirements for global products compare with developing requirements for domestic ones?
Barnes: It is a much more complex process to develop global requirements. You have many more players who desire input. And once you've collected and interpreted the input, you have to go back and verify that you got it right. Normally, we collaborate with individuals we call country managers, the people managing our direct subsidiaries in that country. Their input helps us establish a basic target for a product. Then, when time permits, we get our human factors engineering specialists involved, working closely with representative end-users in selected countries to develop a deeper understanding of their needs.
|
Michael Wiklund |
Wiklund: My clients have tended to focus their human factors research efforts where they have the largest market share. It's a protective approach, and maybe even the smartest approach, but it may be limiting their growth into other markets where consumers have special requirements.
Gruchmann: In Europe, you'll see manufacturers that place countries in clusters based on the similarity of user needs. For example, they will place Germany and The Netherlands in one cluster, Spain and Italy into another cluster, and the Scandinavian countries into a third. So, a manufacturer might conduct research in Germany, Spain, and Denmark, presuming that these countries are fairly representative of many more. Unfortunately, this approach might not reveal nuances in a particular country's healthcare system or hygiene practices, for example, that could have a positive influence on product design. Addressing [Barnes's] earlier comment, I agree that it is a good idea to ask those she calls country managers to help define user requirements. However, I think the information obtained from those people can sometimes be biased or incomplete. For example, nurses in some countries do not always speak openly about the problems they experience with devices. So a country manager might never hear about certain problems. Yet, direct observation of nurses might reveal those problems. So might one-on-one interviews.
Meet the Panel The roundtable was organized and moderated by Michael Wiklund, a frequent contributor to MD&DI and a member of its Editorial Advisory Board. Wiklund is founder and president of Wiklund Research & Design (Concord, MA; www.wiklundrd.com), a human factors consulting firm that specializes in making medical devices safe, effective, usable, and appealing. His recent consulting projects have involved the design and evaluation of dialysis, patient monitoring, and cardiac therapy equipment, as well as several surgical devices. A certified professional human factors engineer, he is also involved in the development of human factors standards for medical devices, serving on IEC's and AAMI's human factors committees. Joining Wiklund in the roundtable were Torsten Gruchmann and Stephanie Barnes. Gruchmann is founder and managing director of Use-Lab GmbH (Steinfurt, Germany), a human factors consulting company focused on the development and optimization of medical devices for usability. He has more than eight years of human factors experience. His recent projects have involved the design and evaluation of equipment used in operating rooms, intensive-care units, and imaging departments. He graduated from Muenster University of Applied Sciences in 1995 with a degree in physical engineering. Since then, he has focused on understanding user needs and translating them into product specifications and concept realization. Before founding Use-Lab, he set up and managed the Center for Usability Engineering and the Center for Biomedical Engineering at Muenster University. Barnes is the human factors engineering manager at SonoSite, a manufacturer of hand-carried ultrasound medical devices. She is responsible for setting the vision for SonoSite's user experience and ensuring user needs are satisfied. She holds an MS in human factors from the University of Idaho and a BS in psychology from Texas State University. As a key member of SonoSite's product development team, she has received several patents and design awards. She has been designing products using a user-centric design process for more than 10 years and focusing on medical devices for the past 6 years. Prior to working at SonoSite, she was involved with a diversity of projects including a military simulation training system, a communication system for air traffic controllers and consumer printers. A passionate advocate for the user, Barnes strives to optimize human performance by following sound practices and a systematic process. |
But country managers are still a solid resource, right?
Barnes: Yes. They get their information straight from our customers. These people, or their direct reports, sell the products on a daily basis. They know what it is like to lose sales because a product does not have a particular feature or because it is too difficult to use. They also get feedback from the people who train customers to use products, as well as [those who] service products. So, while their information may be biased, it still has considerable value. You just have to further validate the information by means of follow-up analysis. Lacking input from country managers, the task of developing global user requirements would take much more time and effort, which is often unacceptable because of a tight development schedule.
Wiklund: Indeed, you can get a black eye by spending too much time and money conducting international research. To outsiders, it looks like you've been on an all-expense-paid international junket while others slog it out back home. Yet, we all recognize the value of international research. So, how much international research is enough before you create the appearance of overdoing it?
Gruchmann: The key is to go where you can get the most valuable input. Some of my clients have focused their research efforts on new markets rather than existing ones where they enjoy solid sales. They feel that meeting new market needs is key to expanding their business. At the same time, they hope that product features developed to meet new market needs might also please their established customers. They sometimes feel they can skip their major markets because they already understand those customers very well.
Is it possible that new market needs might conflict with established market needs? Wouldn't this create a risk of the tail wagging the dog?
Gruchmann: There is a risk, but it is manageable by having major-market representatives check your evolving user requirements. Also, there have been many cases where a manufacturer has developed a product for a niche market, only to find that it has great appeal to the established customer base. This is particularly true of “light” versions of devices that have been defeatured to make them smaller and simpler to use. The general appeal arises due to their enhanced usability, even if they may not be as functionally powerful.
Barnes: Going back to your question about scaling one's research efforts, we collaboratively decide how many and which countries to visit. We base our decisions on where we have current sales and distribution channels, as well as where we want to sell and distribute our products in the future. The collaborative decision making ultimately helps us defend the spending.
Do user-research planners need to be alert to corporate politics?
Barnes: Definitely. The people selling the product need to be involved in the process. You don't want them to feel that you developed a new product and tossed it across the ocean without their prior input. If they are involved in the design process—helping to define user requirements—they are more apt to accept the final design, and it will better fit with their market, even if it does not meet every one of their stated needs.
Let's shift the conversation to some practical considerations regarding international research. How do you make sure your research efforts proceed smoothly? There's a difference between working in Boston and conducting research at several New England sites and conducting research in Helsinki or Shanghai, for example.
Gruchmann: We maintain a database of professional contacts worldwide, so we have people who can help us set up research activities on relatively short notice.
Wiklund: You're suggesting building a strong research network so that it is already in place when you need it.
Gruchmann: That's right. And you want some of your contacts to be local consulting organizations that can quickly conduct the research on your behalf, often for less than it would cost to do it yourself.
How do you make international research activities go smoothly?
Barnes: Again, our country managers can be extremely helpful. But, they sometimes want to bring you to their luminary sites. These sites don't always provide access to representative customers.
What do you mean by luminary sites? That's an interesting term.
Barnes: A luminary site is one that tends to be research oriented and less involved with routine clinical practice. People working at these sites tend to be experts and very influential in their field. They are pioneering advances in their particular clinical applications and are well respected by their colleagues. It is important for a company to consider input from luminary sites, but it can also be hazardous to not broaden your research. Their expectations and needs can be much more sophisticated than at typical sites. They may need and ask for oranges while the larger population needs apples. So we don't drive our products solely based on a luminary site's demands. At the same time, appeasing luminary sites is really important to the adoption of new products. You have to strike a balance.
Gruchmann: We also have experience working with clients who turn to luminary sites, what we call reference hospitals. But biased feedback is a problem. These hospitals and their doctors like to gain control over product development decisions. They also want to be the test site for the new product, and to ultimately purchase products for a lower price. These are factors that can interfere with the collection of accurate user requirements. So we guide our clients to select a wide spectrum of research sites whenever possible.
What other advice can you offer researchers bound for foreign countries?
Gruchmann: It helps to have someone on your research team who speaks the local language. For example, we have a staff member who is fluent in Chinese. This was a great help when we conducted a two-week visit to 10 hospitals all over China to collect user requirements for a new medical product.
Barnes: We don't always have someone on our team who speaks the native language, although that would be nice. But every international trip I've ever made involved a host—a local person who spoke the language and served as our translator. When you are observing product use or conducting an interview, a host can provide supplemental explanations. Face it. You really do not understand a foreign population's culture, its history, the characteristics of competitive products, or the associated systems, such as the billing system. A local host can clue you in to the subtleties.
What are some of the notable differences in the nature of different user populations and their medical device requirements?
Wiklund: For example, I find that caregivers in the United States do not like to read user manuals. There is little you can do to get them to sit down and read about device operation before they try using it. They'll tell you they are too busy.
Can you share your findings about specific cultures as they pertain to product development, perhaps the risk of stereotyping?
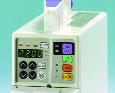
|
The color conventions used on this infusion pump in China vary from those seen in U.S. devices. |
Barnes: We found that medical device consumers in Japan value smaller products, as opposed to larger ones. Their preference may be driven by the fact that the country is congested, and many things are built small to accommodate for the congestion. So miniaturized products are admired. By contrast, customers in China seem to place a higher value on larger products, as opposed to smaller ones. So, these cultural preferences seem to be based on emotional needs and core values, as well as practical considerations.
Gruchmann: I agree. We recently had a discussion with a client from the UK on this very topic. We noted that Japanese hospitals usually have small ORs, so they require smaller-than-usual medical devices. However, in China, the ORs are much larger, so device compactness is not particularly important. In fact, I know of a laboratory device that has been successful in Germany because of its small size. But when the manufacturer tried to sell it in Asia, customers there felt that such a small device should not cost so much. In response, the manufacturer made the device's footprint and housing larger and added a metal plate for weight. The only benefit of the extra size was room for more test strips. However, these superficial changes helped the product achieve success in Asia. As another example of cultural differences, I know that many Germans are quite comfortable learning to use complex devices. It gives us a sense of accomplishment, whereas people in other cultures might regard the same device as unnecessarily complex, absorbing too much learning time.
Many U.S. companies initially introduce a new product in Europe, expecting to introduce it domestically once they receive FDA approval, perhaps months or years later. This leads many developers to question whether there are any profound differences between customers in Europe and the United States.
Barnes: There is a lot of commonality, but certainly there are differences. I would discourage anyone from assuming the two populations are the same.
Wiklund: You hear that Europeans love icons and that Americans hate them. The standard explanation is that the multiple languages spoken in Europe make text labels impractical. As a result, the ubiquitous use of icons—in all walks of life—has trained Europeans to expect and prefer them.
Barnes: We've definitely found that customers in certain European countries prefer symbols. But, as you suggested, we are not sure whether this is because they are accustomed to icons in general, or accustomed to seeing them on competitive ultrasound systems. Our solution is to use icons to label hardware features, but to use icons combined with text to label software features. To support this, we like to do domestic and international testing of our icons to ensure comprehension and to make sure that they do not offend potential users.
|
Field research in The Netherlands showed that users had to add their own label to clarify this scanning device's on and off buttons. |
Gruchmann: I agree. You have to use symbolic labels on European products because of the language problem. But we Europeans actually dislike symbols because so many are not intuitive. In my opinion, it is difficult to develop symbols that are clear to customers in every market. Cultural differences have a strong influence on how people interpret icons. Software menus can be used to explain symbols, or soft keys can be used instead of symbolic, labeled hard keys.
Barnes: Sometimes we intentionally use symbols that are quite abstract, as opposed to representational or familiar. This approach helps to avoid negative associations and critical confusions. We also try to limit our icon library to the fewest number possible.
If you were under tremendous time pressure—i.e., did not have the time to launch a multicountry research effort—and had to take a quick-and-dirty approach, how would you gather user requirements for a global product?
Barnes: If I need information quick, I e-mail a survey to the country managers. The process usually takes a couple of days by the time I send it out and hear back from everybody. I'll also conduct phone interviews with current customers. They are really easy for me to identify and contact. I might also conduct a Web-based survey. So I usually try to attack the problem from several directions.
What is your approach to conducting Web-based surveys?
Barnes: There are some great services for which you pay by the question. They have a lot of experience hosting surveys, compiling the data, and getting the results back in short order. But, you still have to recruit the right people to complete the survey. So again, if I am doing something quick and dirty, I will leverage our current customer base. Current customers are much more likely to participate in the survey than noncustomers. If our study concerns cardiology, I simply pull from our database of cardiology customers.
Wiklund: In my company, we've had success sending software user-interface prototypes to prospective users via e-mail. Recipients can run the prototypes in a standard browser. Using Web conferencing tools, we can direct a pretty effective walkthrough or even a full-blown usability test without leaving the office.
Can you cite cases in which international research has taught you something key to a particular product's success?
Gruchmann: We conducted a study of infusion techniques at several sites across Europe and discovered significant differences. In Germany, we found that nurses do not calculate infusion rates, but rather keep the appropriate values in memory. By comparison, we found that nurses in southern Europe and in the UK set the volume to be infused over a period of time and leave it to the pumps to calculate the infusion rate. If we limited our investigations to Germany, we would have overlooked an important customer expectation.
Barnes: Our ultrasound systems also incorporate a calculation package. After users acquire an ultrasound image, they might use various tools to determine certain dimensions. Then, the software will derive additional measures. If we focused solely on the Untied States, our products would probably be unacceptable in many international markets because users in those markets count on different calculations to make diagnoses.
Wiklund: Working with an anesthesia equipment manufacturer, I found significant differences in anesthesia delivery in Denmark versus the United States. In Denmark, a nurse and a physician work together as a team to deliver anesthesia. By comparison, an anesthesiologist or a nurse anesthetist in the United States works independently. This difference had significant implications in terms of display readability.
When you plan to conduct international research, how do you decide how many subjects to involve at each site?
|
Scenes from user research conducted at comparable CT scanning facilities in the United States (left) and China (right). Design teams should try to replicate conditions when conducting research in other countries. |
Barnes: I do not think I've ever been completely comfortable with our sample sizes, which tend to be small. It seems you can always learn more by talking to more people. But the logistics of overseas studies can be challenging for us. Often, when I am conducting a usability study in a foreign country, I might visit only one person a day because of the time absorbed by travel.
How much time will you spend with each individual?
Barnes: No more than half a day. I usually go to the customer, rather than bringing customers to a central research facility. It allows me to conduct observations and interviews in the context of product use. However, if we are concerned about reaching more participants in an efficient way, we might conduct our research in conjunction with technical conferences. This method enables us to prescreen participants that we meet on the conference floor to be sure they have the right background to address our research interests. We'll have properly qualified participants come to a nearby hotel suite to participate in an interview or test.
If you can draw a sample of prospective customers from various countries at a conference, is that a better or worse alternative to conducting research in those countries?
Barnes: It is an expedient alternative if we are evaluating our device, rather than trying to observe and learn about their environment and work flow in their natural setting. For example, at the American Heart Association's recent national conference, I was able to talk to a sample of about 20 cardiologists from Japan in just two days.
Is a 20-participant sample typical of most research activities?
Wiklund: Regarding international research efforts, usability testing in particular, I assume a sample of 10 or so participants would be sufficient. Doubling the sample size might improve the face validity of the findings, but is not likely to uncover many more usability problems, particularly major usability problems that become evident after the first few test sessions. In short, you can learn a lot from a small number of people. You don't have to make your studies enormous.
Gruchmann: I agree. When we are conducting international studies of intensive-care products, for example, 10 people are certainly enough. We might increase the number of participants if the target user population is much larger—like the one for consumer products—or when it includes children and elders or other special populations.
Is usability more important in some markets than others?
Barnes: One of my company's core pillars—in fact, its guiding philosophy—is ease of use. We took an unwieldy technology that required you to have a PhD in “knobology” to operate it, and made it portable and easy to use. We simply assume that customers worldwide will appreciate ease of use, usability, and intuitiveness of the product. We don't question the value of usability.
Your philosophy is to invest in usability, presuming that it will pay off for for SonoSite as well as its customers?
Barnes: Right, because we see usability as a prime differentiator between us and other ultrasound manufacturers.
Wiklund: U.S. consumers may have led the demand for user-friendly products, at least from a software interaction point of view. And years ago, Europeans seemed to be more sensitive to device ergonomics. However, there is now worldwide demand for usable software and hardware user interfaces, fueled in part by the availability of excellent consumer products.
Gruchmann: I agree. Specific markets might pose different feature requirements, but there are no appreciable differences in the need for usability. Usability has become a core requirement of products intended for the global market. But that is not to say that every manufacturer is optimizing its products for usability. Some have a long way to go.
Barnes: You cannot invest heavily in usability at every stage of design. And you cannot answer every usability-related question by conducting an international field study. You have to decide where in the product development cycle you want to invest in international research—for testing, in particular. Then the challenge is to get as much out of the effort as possible, sometimes conducting research on several products at a time.
Wiklund: Fortunately, it is a lot easier to conduct international research than you might expect. The key is to plan ahead and set aside the necessary funds, confident that there ultimately will be a big payoff.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like