Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published February 2001STERILIZATIONThe ability to accurately monitor EtO levels throughout the sterilization process is expected to facilitate validation and result in more cost-efficient operation.Patrick G. Smith
February 1, 2001
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published February 2001
STERILIZATION
Patrick G. Smith
 Widely used for sterilizing medical equipment, as well as cosmetics, pharmaceuticals, and food products, ethylene oxide (EtO) has proved to be economical, available, and efficacious for eliminating bacterial and viral microbes. Pumped into a closed container, EtO can effectively eliminate microorganisms within an acceptable time frame.
Widely used for sterilizing medical equipment, as well as cosmetics, pharmaceuticals, and food products, ethylene oxide (EtO) has proved to be economical, available, and efficacious for eliminating bacterial and viral microbes. Pumped into a closed container, EtO can effectively eliminate microorganisms within an acceptable time frame.
Despite its effectiveness as a sterilant, however, EtO has a number of significant drawbacks. It is highly toxic—even a sniff can be fatal. EtO can also be dangerously explosive in concentrations as low as 3% by volume, or 30,000 ppm—a spark in the presence of just a trace of the gas can pose a serious hazard. And because EtO carries its own oxygen supply, it is also flammable at 100% concentrations. Put simply, it can explode even in anaerobic atmospheres.
The effectiveness of EtO sterilization systems is largely dependent on several factors, including gas concentration, diffusion rate, temperature, and humidity. Each of these process elements can either directly or indirectly influence the system's level of microbial lethality. Each factor must also be balanced against any limitations that may become apparent with the use of specific packaging or products that are to be sterilized with the system.
Gas Concentration. Generally, as EtO levels increase, the sterilization process becomes more effective and requires less dwell time. Concentrations in the chamber are limited by Ideal Gas Law parameters owing to potential condensation.
Diffusion. Another way to reduce dwell time is to increase the diffusion rate of EtO from the chamber to product in the load. This can be accomplished by creating a vacuum in the chamber before it is charged with EtO. As the EtO is injected into the chamber, pressure difference effectively "pulls" the EtO into the product. Another benefit of reduced pressure is increasing the concentration at which condensation occurs. This allows higher EtO concentrations during the cycle.
Because creating and maintaining a high vacuum can be difficult, time-consuming, and expensive, some chambers have been designed to operate with a partial vacuum or at normal air pressures. Using any vacuum, however, can result in certain product-related problems. Some products, for example, may be sensitive to any negative air pressure in the chamber.
Temperature. Because EtO liquefies at 51°F at STP (standard temperature and pressure), the temperature levels inside the sterilization chamber must be high enough to ensure that the EtO is a gas. Thus, product tolerance for high temperatures is another militating factor because chamber temperatures can range from 100° to 150°F, depending on the product to be sterilized. This will also affect sterilization times and efficiencies.
Humidity. The presence of humidity is believed to increase EtO penetration. Thus, maintaining a wet atmosphere in the sterilization chamber can increase the effectiveness of EtO sterilization. A relative humidity (RH) of 35–90% has been demonstrated to be beneficial for effective EtO sterilization. Water vapor, usually steam, is injected before EtO to shorten the time required to complete the sterilization process. The presence of increased humidity, however, can cause the formation of condensation on the product, the chamber walls, and optical EtO sensors.
|
All these variables—as well as such other factors as product shape and absorption—have made monitoring and controlling the sterilization process imprecise at best. As a result, it has become common for EtO users to add extra time, extra heat, and extra EtO to the process cycle to ensure that satisfactory results are achieved.
Because there has been no precise method of measuring the actual proportion of EtO in the chamber, control of the sterilization process often has been more a function of experience than of instrumentation. Although systems capable of generating fairly accurate EtO measurements have been developed, a number of shortcomings have been associated with the use of these EtO sensors. Currently available EtO-sensing systems include:
Near Infrared (IR) Optical Devices. Although EtO absorbs very well in the near IR, so does water, which in vapor form or condensed, typically causes inaccuracies and sometimes erratic signal variations in these systems.
Electrochemical/Solid-State Sensing Cells. Such systems are effective and accurate in an ambient sensing environment; however, they exhibit water response, pressure response, and high drift and short life owing to the in-chamber environment.
Sample-Draw IR Devices. These systems provide accurate results because they measure samples in a controlled external environment. In addition to being costly and complex, they pull toxic and explosive EtO-drenched atmospheres out of the chamber. Leaks into the room are potentially disastrous.
The challenge has been to develop a system that can measure an elusive and ever-changing EtO ratio yet is impervious to the deleterious effects of steam, condensation, varying vacuum or pressure levels, and highly corrosive atmospheres. Ideally, the sensor would be capable of quantifying actual EtO levels throughout the entire sterilization cycle. the device should also be able to monitor and verify gas concentrations, show absorption profiles for the specific product being sterilized, and ensure that all toxic gases have been evacuated before the chamber doors are opened.
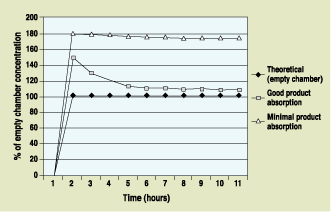
The proportion of EtO at work in a sterilization chamber varies with time and the specific material to be sterilized. Theoretical chamber concentrations are calculated using gas weights, the assumption of an empty chamber, and the application of the Ideal Gas Law. The concentration of EtO that actually reaches the product in the load is generally a function of the load's absorptive properties and the packaging used. If the material does not absorb EtO—as in the case of metallic medical instruments—the initial inrush of EtO causes an immediate peak and the concentration remains high for the duration of the process.
| Figure 1. In-chamber EtO concentration as a function of product absorption qualities. |
Figure 1 demonstrates the changes that occur in EtO concentration levels as a function of product absorption. If the material is absorbent—as in the case of cloth garments or bandages—the EtOpercentage peaks, then drops back as the gas is absorbed. The level finally equilibrates at a concentration that is usually close to the theoretical concentration. Conversely, as the gas is flushed from the chamber, the rate of EtO desorption from the product material is also a function of load composition.
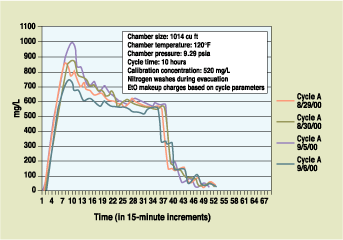
Figure 2. Actual EtO concentration versus time for same-cycle and different loads. |
|
Reading actual EtO concentrations throughout the entire sterilization cycle can be critical. By continuously and accurately measuring the EtO levels throughout the cycle, a sensor can monitor actual absorption profiles, and thus ensure sterilization effectiveness.
It is important to note that the EtO rate is not usually constant. Variations may occur because of the specific product material, temperature, humidity, nitrogen level in the atmosphere, and product silhouette. Variations may also be associated with the specific product covering. Products covered with plastic, for example, would display different sterilization profiles than products covered in foil or some other material.
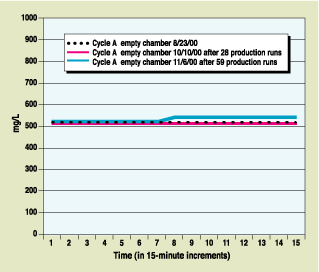
| Figure 3. Actual EtO concentration versus time for same-cycle and empty chamber. Chamber size: 1014 cu ft; chamber temperature: 120°F; chamber pressure: 9.29 psia; calibration concentration: 520 mg/L. No adjustments, recalibrations, or modifications were made to the SEC EtO monitor during the entire time period. |
To address the needs specific to the use of continuous EtO monitoring, a sensor has been developed by Sensor Electronics Corp. (SEC; Minneapolis). The multiwavelength IR light–based sensor is designed to continuously measure the hydrogen-carbon bond IR light absorption of EtO molecules during the sterilization process.
Like certain conventional sensors, the new design uses IR absorption technology to precisely define and measure EtO levels throughout the sterilization operating cycle. The sensor, however, has been designed to remain unaffected by saturated RH levels and resulting condensation on the sensor viewing lens. Figures 2 and 3 illustrate in-chamber EtO concentration as measured by the sensor. Figure 2 shows the results of monitoring for loads composed of different materials; Figure 3 indicates the device's sensing accuracy by comparing three empty chamber runs in order to verify the calibration point.
The detector uses IR levels to quantify the actual EtO concentration in the sterilizer chamber. The proprietary method uses principles founded in molecular physics. IR radiation at specific wavelengths excites certain molecular resonances. In the process, some IR energy (considered as light) is absorbed, with the amount of absorption being a function of the concentration, as illustrated in the equation:
About the Author(s)
You May Also Like