Continued growth in medtech’s contract manufacturing segment could have implications for its medical device clients.
July 1, 2007
BUSINESS PLANNING & TECHNOLOGY DEVELOPMENT
|
In spite of mountingoperating expenses, themedical device industryhas experienced substantialgrowth duringthe past decade. Medtechcompany leaders have managedto maintain companyand industry profitability bysupporting an extraordinarilyhigh level of commitmentto product innovation and byadopting a variety of creativecost-cutting measures--including the increasinglypopular strategy of outsourcedmanufacturing.
Structural factors withinthe device industry have createda pivotal role for companiesin the contract manufacturingsegment, generating significantattention from a diverse but relatedgroup of stakeholders--includingincumbent market leaders, emergingdevice manufacturers, and privateequity groups. Such increased attentionfrom various stakeholders is drivingopportunities for the continuedgrowth of businesses in the segment.Ultimately, consolidation among themany outsourcing companies thatare operating in the medical manufacturingsector is expected to occur.
Market Growth
Contract manufacturers have certainlybenefited from the growth ofthe medical device industry. However,growth of the outsourced medicalmanufacturing segment is outpacingthat of the device industry asa whole, indicating a trendtoward the increased use ofoutsourcing.
Al though approximationsof the market size foroutsourced medical devicemanufacturing remainimprecise, estimates indicatea U.S. market size of roughly$4.4 billion as of 2005--withan estimated 20% of all medicalequipment productionoutsourced to third-partyvendors--up from a marketsize of $2.2 billion in 2002. By2010, it is projected that anestimated 40% of all medicaldevice manufacturing couldbe outsourced, representinga 15% annual growth rate over thenext few years.1
Industry Drivers
A variety of factors are drivinggrowth in the contract manufacturingsegment. Intense competitionhas forced device manufacturers tofocus on their core competencies ofresearch and development (R&D), clinical education, marketing, andsales.
One major factor driving thegrowing trend toward outsourcingmanufacturing operations is theneed to reduce direct expenses andstreamline supply chains while offsettingescalating operating expenses.The medtech industry has beensuccessfully improving its gross marginsthrough better productivity andmore-effective product mix andmanagement. These margin improvementsare under constantpressure from competitive productsand reimbursement rates. With pressureon margins, time to market formedtech products is a critical consideration.With a limited patentprotection window, any developmentor manufacturing delays canbe costly.
Another primary driver of thecontract medical manufacturing segment'sgrowth consists of not onlythe expansion of the overall deviceindustry, but also the extent to whichdevice manufacturers are choosingto outsource their manufacturingoperations. Although the overallmedical device industry in the UnitedStates is expected to continuegrowing at a strong rate, the outsourcedmanufacturing market isprojected to see even faster growth.As medical device manufacturersstrive to improve margins and timeto market, outsourced manufacturingwill continue to grow in acceptance.1
Strategic Rationalefor Outsourcing
Contract manufacturing has a number of strategic benefits beyond simply reducing product acquisition cost. Incentives for device manufacturers to outsource their production include the following.
Quicker, More-Efficient ProductLaunches. Engaging full-service contractmanufacturers in the design anddevelopment phase of a product's lifecycle can do much to condenselaunch timelines. Furthermore, manufacturingexperts working in conjunctionwith product designers arebetter able to achieve productivitygains by implementing design-formanufacturetechniques and drivingout production inefficiencies over thelong term.
Ability to Focus on Core Competencies. Medical device manufacturersearn greater returns on capitalby investing in product developmentand marketing than in manufacturing.Establishing manufacturingcapacity is both capital intensive andslow going, resulting in a lower returnon investment over a longer periodof time.
Access to Specialized Capabilities. Contract manufacturers areable to become experts in certainniches in the market, allowing manufacturersthe option to use thesespecialized production capabilitieswithout spending valuable R&D dollarson projects that distract fromtheir core competencies.
Flexibility of Production. Contractmanufacturers are able to usetheir capacity more efficiently basedon a consistent flow of orders. Theycan better accommodate large orderswhen needed.
Keeping Projects In-House
Although the argument for outsourced manufacturing is compelling, there are instances in which manufacturers choose to handle projects for which they would otherwise rely on contract manufacturers. Such reasons include the following.
Surplus Capacity. Given thefixed-cost nature of manufacturing,manufacturers may look to leverageunderutilized resources before outsourcingwork.
Manufacturing Control. Devicemanufacturers may keep projects inhouseto more efficiently implementmidstream process changes or to protecttheir proprietary processes ortechnologies. This occurs on a caseby-case basis, given that many largeroutsourcing partners are becomingtightly integrated into the overall supplychain, including codevelopmentof products.
Liability Issues. Device manufacturersare ultimately liable forproducts they market. There may beinstances, such as intense inspectionof a particular product line, that couldnecessitate ownership of the manufacturingprocess.
Market Share Breakdown
With more than 3000 individualfirms providing a wide range of outsourcedservices to medtech companies,the contract manufacturing segmentis highly fragmented. Thesefirms have very different backgroundsand capabilities, and include everythingfrom small owner-operator machine shops that used to manufactureaircraft parts to professionallymanaged, private-equity-backedproviders of end-to-end services. Thesize of the market and the variety oftasks that can be outsourced--design,machining, stamping, assembly,inspection, sterilization, and packaging,to name just a few--enable thisbroad spectrum of providers to coexistsuccessfully.
|
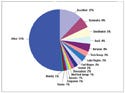
Figure 1. Contract manufacturing market share in 2005. Source: Millennium Research, Frost& Sullivan, company reports. |
Approximately 50% of the contractmanufacturing market formedical devices is controlled by nomore than 12 firms, with leaderAccellent Inc. (Wilmington, MA)controlling an estimated 12% of themarket. Beyond the 12 leading firms,the other 50% of the contract manufacturingmarket is highly fragmented,comprised of firms thathold less than 1% market share each.Such a market structure effectivelyestablishes two tiers of participants:large full-service organizations andsmall niche service providers (seeFigure 1).
Fully integrated manufacturersprovide a wide range of services todevice manufacturers that mayinclude everything from design andmanufacturing to packaging and distribution.Niche providers generallyprovide one specific task, such ascomponent manufacturing, assembly,or packaging. Due to the growth ofthe overall market, both types of companiesare thriving.
Full-service firms provide a numberof advantages to medtech companies.The primary advantage is theease of management. Rather thanworking with a complicated networkof disparate service providers, devicefirms have one partner. This alsoenables better coordination of servicesthroughout the entire process.During the design phase, manufacturingand packaging considerationscan be fully taken into account by theorganization that will be responsiblefor those activities. Such an arrangementcreates a faster time to marketand a more efficient process.
Full-service outsourcing is notwithout its disadvantages. To be successful,it requires careful coordinationand communication among allof the processes. Fully integratedproviders that are unable to providesmooth coordination of theseprocesses negate many of theiradvantages. It is also difficult for amedical device company to find aone-stop shop that is an industryleader in all services. Full-serviceproviders may lack specific capabilitiesrequired by a medtech company.Certain materials and componentsmay require specialized manufacturingand packaging that only a handfulof companies can provide. Inthose cases, relying on a full-serviceprovider that lacks certain specializedcapabilities may cause compromisesto the product.
Niche providers enable amedtech manufacturer to assemble abest-in-class outsourcing programby selecting the service providers thatbest meet the needs for each step.These providers give a developer themost flexibility in manufacturingand packaging alternatives. However,it goes without saying that as a firmuses more outsourcing partners,more oversight and coordinationefforts will be required. The user isthus open to more risk should anyone part of the outsourcing chaindevelop difficulties.
Outsourcing strategies for medicaldevice companies take severalforms. Many companies make theirfirst foray into outsourced manufacturingwith a single project. This maybe a low-volume project for the manufactureand assembly of a device thatis undergoing clinical trials. Often,the medical device company may notwant to make the investment inmanufacturing capabilities until theproduct has been approved. Onceapproved, the project may remainoutsourced or be taken back in-house.
At the other end of the spectrum,medical device companies maychoose to outsource the completedesign, manufacture, assembly, and packaging of a product. In such cases,medical device companies work closelywith their outsourced partners anddo not have to make any in-houseinvestments into these processes.Some medical device companies, inattempting to reduce their investmentin manufacturing and packaging,have sold entire plants to outsourcingproviders.
Outsourcing, when done properly,requires much less capitalinvestment in processes and leads tofaster development and productiontimes. However, medical device companiesassume the risks associatedwith taking core processes outside oftheir control by trusting third partiesto deliver. The partnershipsbecome even more important whencertain proprietary technologies orprocesses are provided to the outsourcingpartners.
In today's marketplace, manymanufacturers are using a combinationof the aforementioned tactics.Certain firms still do significant workin-house and outsource select functionsto niche providers. As theseorganizations seek to outsource morefunctions, they either move toward afull-service manufacturing provideror start to develop their own outsourcingnetwork to provide the additionalservices. To the extent that theirexisting suppliers have added capabilities,they will be the likeliest candidatesfor the new business.
Consolidation amongContract Manufacturers
The growth and fragmentationof the overall contract manufacturingsegment and the demand formore full-service outsourcingproviders will inevitably lead to consolidation.As full-service providerslook to gain additional capabilitiesand market share, they will accomplishthis in part through acquisitions.Niche providers will also seekselected acquisitions to broaden theirmarket presence and capabilities. Ingeneral, some consolidation will beadvantageous for both the contractmanufacturing segment and its medicaldevice clients.
From the medical device companies'perspective, having to work withfewer suppliers is beneficial. The highdegree of fragmentation within thecontract manufacturing segment canmake it more difficult for medicaldevice companies to find and managethe best solution. Currently, a number of medical device companies areactively trying to shrink their lists ofsuppliers.
The outsourced medical manufacturingsegment will also benefitfrom some consolidation. Havingfewer, larger companies will help tobetter define the industry and make iteasier for medical device firms toimplement outsourced solutions. Inaddition, consolidation will attractnew capital and expertise into the segmentas it becomes more attractive toprivate equity firms and larger experiencedoutsourcing firmsfrom other industries. Thisadded capital and expertisewill enable contract manufacturersto serve medicaldevice companies withadditional services andcapacity.
Given the highly fragmentedstate of the contractmedical device manufacturingsegment, there isplenty of opportunity forconsolidation. A recentexample is Symmetry Medical'sacquisition of TNCOInc. (Whitman, MA). Whilefocused mainly on themanufacture of surgicalimplants and instrumentsfor the orthopedic devicesector, Symmetry Medicalalso serves the dental,osteobiologic, and endoscopy sectors.Symmetry aims to be a one-stop shopfor all its customers' contract manufacturingneeds. Its latest acquisition,TNCO, specializes in designing, engineering,and manufacturing instrumentsfor arthroscopic, laparoscopic,nasal sinus surgery, and other minimallyinvasive procedures.
With a market share of 6%, Symmetryis the second-largest player inthe medical device contract manufacturingmarketplace. In comparison,TNCO is one of the smaller players inthe segment, holding a less-than-1%market share. Although TNCO hadachieved success with a strong portfolioof patented designs, it lacked thesize to compete with larger players inthe crowded segment, as is the casewith many of the small companiesthat make up the contract manufacturingsegment. TNCO's strong businessbut lack of resources made it aprime target for acquisition by a largerstrategic player that could maximizeits potential.
Consolidation of the two companiesopens the door for themerged firm to exploit economies ofscale and other synergies to decreaseoperating expenses and increasesales. With Symmetry's larger scaleand re sources, the combined entitywill be able to cultivate and expandon TNCO's intellectual capabilitiesand grow more quickly and effectivelyin TNCO's fields of specialty.Symmetry's acquisition of TNCOwill enable the combined entity toexpand the scope of its business andincrease its market share. Much likewithin the medical device industryas a whole, these types of buyoutbenefits will continue to drive themedical device contract manufacturingsegment toward a more consolidatedfuture.
Conclusion
Medical device companies willcontinue to have a wide range ofoptions in how they take advantageof outsourcing services to reduce theircosts, capital investments, and time tomarket. The breadth of suppliersproviding everythingfrom component designand manufacturing to customizedpackaging servicesprovides medical devicecompanies with the opportunityto customize theiroutsourcing program.
The market for outsourcedmedical manufacturingis growing rapidly.The combination of thisrapid growth with a highlyfragmented market has createda ripe opportunity forconsolidation. This consolidationwill help the segmentto grow by attractingnew capital and expertise,and allowing outsourcingfirms to offer a wider rangeof coordinated services. Inthe end, medical device companieswill benefit from the creation of astable group of outsource suppliersthat can provide flexible solutions tomeet whatever needs they require.
Reference
1. B Dunn and J Finn, A Strategic Review of Outsourced Manufacturing for Medical Devices (Boston: Covington Associates, 2007).
Ben Dunn is managing director of Covington Associates LLC (Boston), a specialty investment banking firm.
Copyright ©2007 MX
About the Author(s)
You May Also Like