Originally Published MDDI April 2006MDEA 2006 MDEA 2006 showcased exciting devices that have taken several steps forward in design, safety, and usability. Heather Thompson
April 1, 2006
MDEA 2006
It is difficult to talk about innovation without using comparative terms (as in faster, safer, better). After all, rewarding a product that improves delivery time, or that includes a locking system so patients can take it home, is what the Medical Design Excellence Awards (MDEA) are all about.
So when you see a device designed to reduce use error, suture with less damage to tissue, or stay cool more efficiently, you'll understand the effort that goes into improving such a product.
It is difficult to take a device that could use improvement and meet design challenges to make necessary changes. Sometimes it takes more imagination to rethink a device to make it better than it does to come up with a completely new product.
That's why the following devices come out on top. They have each greatly improved the way things are done. And it's a sure bet the manufacturers are already looking at these award-winning devices to see where they can make advances—and the products are getting better all the time.
|
Patton Surgical's PassPort Shielded Trocar addresses safety concerns and does not interfere with surgical methods. |
Getting Safer: Injury Reduction
PassPort Shielded Trocar. Injury reduction is one of the most important aspects of surgery. Sharps that can hurt patients and pose risks to doctors and nurses are continually being examined for ways to reduce the chance of injury.
The trocar has significantly changed medicine. Used in laparoscopic operations, a trocar makes the first puncture to allow surgeons to perform image-guided surgery. The minimally invasive procedure means a quick, painless operation, shorter recovery time, and less scarring. It is a decidedly useful device.
The problem is that trocars are by necessity very sharp devices that are driven into the body by a surgeon who can't yet see below. The method creates a risk because the surgeon could hit an artery or an organ, causing serious and sometimes fatal injuries in patients. Michael Patton of Patton Surgical (Austin, TX) says that trocars are not yet at the same level as other laparoscopic devices. “Trocar technology has not advanced at the same pace and with the same sophistication as other laparoscopic instrumentation. Trocars are the common denominator in laparoscopy, so change was imperative.”
Attempts to create safe trocars have yielded mixed results. Some trocars rely on a spring-loaded sheath to deploy over the sharp edge once the device has been pushed through skin, fat, and connective tissue. But the shield may not always deploy fast enough, or it may get caught on tissue and not deploy at all. Surgeons may also break the shield by pushing too hard.
The PassPort Shielded Trocar by Patton Surgical has incorporated several design features that attempt to make the trocar safer. Based on a method created by Harrith Hasson, PhD, the PassPort uses a sharp blade to cut through connective tissue, fat, and skin, but then uses a shield over the blade to create a blunt piercing object for the last few millimeters. Patton says that the company strove to meet certain design challenges so that surgeons would feel comfortable with the device for differing use methods. “Some surgeons prefer a trocar with a sharp blade for a more-controlled feel,” he says. “Others choose bladeless trocars because they are concerned about the sharp blade. The main challenge was creating one trocar that satisfies the needs of all surgeons.”
The device has a shark-tooth-shaped blade that enables the tip shield to ride down the center of the obdurator. This means the tip and blade are fitted into obdurator shells and are welded together. Fewer parts and dual protection mean the shield can deploy more quickly in a limited space (down to 5 mm). The shield deploys before it breaks through the cavity, enabling blunt penetration.
As Patton explains, shield indicators on the proximal end of the device tell the surgeon where the shields are in relation to the blade. “The PassPort provides the surgeon with both audible and visual signals to indicate where the shields are at any given time during insertion,” he says.
First, the surgeon hears a click when the tip and blade shields are retracted, exposing the cutting blade. Additional clicks sound when the tip and blade shields advance to cover the cutting tip and blade edges, respectively. Shield indicators on the side of the obdurator display whether the trocar is in cutting mode, and display the position of the tip and blade shields relative to the blade.
MDEA jurors were impressed with the trocar's design. Yadin David, director of biomedical engineering and television services at the Texas Children's Hospital (Houston), was particularly interested in the design and safety mechanisms. “Trocars are being scrutinized for the injuries they cause, and I paid particular attention to this when reviewing the device,” he says. “I liked the innovation that was built right into the device, with the double defense mechanism. The instrument provides a new approach.”
360° Fascia Closure Device. In addition to devices that protect patients, sometimes products need to protect those performing surgeries. According to the American College of Surgeons, 59% of all needlesticks occur during fascia closure. Nurses and doctors are at a high risk for needlestick injuries, and great strides have been made to reduce that risk. The best devices combine a reduced risk for needlestick while also improving the method for use.
|
The SuturTek 360° Fascia Closure Device introduces needlestick prevention methods and is designed to reduce hand fatigue for surgeons. |
Such is the case for the SuturTek 360° Fascia Closure Device from SuturTek Inc. (North Chelmsford, MA). The SuturTek device is an engineered sharps-injury-prevention device that complies with OSHA regulations and federal and state needlestick prevention and safety laws. Gerald Brecher, president of SuturTek, explains that the company is the only one to receive 510(k) approval for the claim that the device is for the prevention of needlestick injuries, but he thinks that other companies may soon follow suit.
The device comprises a reusable needle driver and a disposable suture cartridge that protects the user from the needle. The needle driver is an ergonomically designed handle that uses a gear-drive mechanism that transfers two hand squeezes of a trigger-like actuator into a 360° rotation of the suture needle through the tissue. The suture cartridge has a preloaded suture needle bent into 3¼4 of a circle and has suture material swaged into its tail. These needles, Brecher explains, can be attached with any type of standard suture material: braided, monofilament, absorbable (dissolving), or nonabsorbable. According to Brecher, the design mimics surgical needles already in use. “Our technology is designed to replicate the way surgeons suture. And surgeons suture with curved needles.”
Another benefit of the device is that the design enables the needle to follow its own arc, meaning the needle does not change course, which reduces the chance of further damaging tissue as it is being sutured.
Employing the device inhibits hand fatigue during an operation, because the procedure only requires two hand squeezes, rather than the manual grasping, repositioning, and release of hand suturing. MDEA juror David says that he was intrigued by the ease of use presented by the device. “It is straightforward. You don't need to learn a new technique or skill, but a surgeon will probably have more-consistent closures using the device.”
The company worked closely with surgeons and nurses to develop the device, and Brecher is adamant that such a relationship is critical for creating quality surgical devices. “We have an active surgeon advisory board and we tested our prototypes with groups of OR nurses as well. The devices need to be easy to use and easy to learn to use. Only customers can tell you if you're on the right track,” he says.
Brecher explains that SuturTek will continue to redesign surgical needles to increase safety, building on the technology platform of the fascia closure device. “Our first product is the 360° Fascia Closure Device, designed for closing large open surgical wounds. Our next product will be for closing the sternum (bone) following open-heart and other thoracic surgery procedures.”
In addition, Brecher says the company will look at other applications. “We have built and are testing smaller versions of the device for other types of closure, as well as minimally invasive and intraoperative surgical applications.”
|
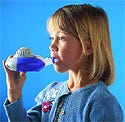
The eFlow Electronic Nebulizer by Pari GmbH delivers required medications quickly and is child friendly. |
Getting There: It's All in the Delivery
eFlow Electronic Nebulizer. Aerosol medication delivery is a popular method for diseases that require pointed drug delivery, especially to the lungs. As a rule, however, the design of these life-saving devices still need much improvement. Nebulizers are bulky and inefficient. With a commonly used nebulizer, administration of drugs that treat asthma, chronic obstructive pulmonary disease, cystic fibrosis, pneumonia, and other diseases can take anywhere from 8–30 minutes. And, as the drugs often require several administrations per day, patients often complain about inconvenience.
With a new delivery technique in hand, Pari GmbH (Munich, Germany) decided to tackle the various problems presented by nebulizers. And the company's efforts are impressive. “This nebulizer is a very friendly product, especially for children,” says MDEA juror Mary Beth Privitera, assistant professor, biomedical engineering, in the medical device innovation and entrepreneurship program at the University of Cincinnati. Privitera says the eFlow allows patients more portability. It is also less intimidating than other nebulizers on the market, she says.
The eFlow Electronic Nebulizer features a vibrating membrane that concentrates the drug and reduces waste as the drug is aerosolized. Concentrated delivery means a 2–3× reduction in administration time. But the change is so dramatic that it is difficult to compare. That is, a drug that usually takes 8–30 minutes to administer may only take 15–20 breaths with the eFlow.
Designers also spent time with the shape and feel of the product. “It is easily packed, stored, and assembled with good design resolution in regard to the ergonomics of medication delivery and aesthetics,” says Privitera. The eFlow is a portable device that can run on batteries or a car charger. The designers gave the device a modular structure that is lightweight and promotes intuitive use. According to its submission, the team spent considerable effort designing audible sounds and lights to guide patients through use. They also created a special mouthpiece to position the device properly.
According to the company, the focus on human factors design combined with increased delivery efficiency will give patients greater incentive for compliance. Juror William Hyman, a professor in the department of biomedical engineering at Texas A&M University in College Station, TX, says “This improves delivery and compliance and reduces lifestyle interruptions. It combines an advance in underlying technology with an attractive and user-friendly unit.”
The design is far from finished, however. “One challenge with the product is that you must pour the medication into a rather small target,” says Privitera. “This was noted in their submission very honestly, as were other potential improvements.” The company has performed several user surveys and will no doubt continue to improve the device based on those results.
|
Delphi Medical Systems Corp. designed the IVantage Ambulatory Infusion Pump to be useful for patients at home. |
IVantage Ambulatory Infusion Pump. Items that are critical in hospitals can often make a transition to other sectors of healthcare, such as in ambulances or even homes. But often, significant changes must be made first to create devices suitable for those environments. An ambulance has limited space, for example. So a device meant for paramedical use must be smaller than one used in a hospital. Furthermore, in the home, devices must have error-proof mechanisms so that untrained patients can use them easily and safely.
Delphi Medical Systems Corp. (Troy, MI) made the IVantage ambulatory infusion pump with various improvements for both ambulances and homes. The infusion system is small. Using an EC 32 flat motor, the whole unit weighs only 13 oz.
One of the first items developed by the company, the IVantage incorporates various safety features. These include a locking system for home use, an anti-free-flow slide lock, and a proprietary pump that only goes one way. The anti-free-flow feature employs a mechanism that ensures that the caregiver engages the clamp to place the cassette on the pump. The same mechanism is also employed to take the cassette off of the pump. According to John Harris, business development manager at Delphi Medical Systems, free flow can be most problematic during removal and replacement of the cassette.
“This is a nice design,” says juror Tor Alden, principal of HS Design Inc. (Gladstone, NJ). “The disposable cartridge is smart, with a nice dosage range. It is a good product.”
Harris also explains that the disposable cartridge plays a significant role, both for safety and for ease of use. “The pump cartridge, or cassette, was designed with the intent to place most of the moving parts of the system into the disposable. Thus, the pump itself requires less maintenance, as it is mostly comprised of electronics,” he says.
The company's goals included safety, explains Harris, in that it brought about the greater goal of creating a usable and valuable product. “Our first and foremost concerns have been on reducing time and trouble, or benefiting the bottom line. Naturally, safety plays a large role in reducing time and trouble, but it can also have a huge effect on the bottom line.”
|
The NanoCool Shipping System employs a self-cooling mechanism to regulate the contents to specific temperatures during transit. |
NanoCool Shipping System. Sensitive medical products often need to remain at specific temperatures during storage. Juror Hyman says, “The NanoCool is, dare I say it, cool. It addresses the need to ship materials under refrigerated conditions in an innovative, easy-to-use, self-cooling system.”
According to John W. Hoffman Jr., marketing director for NanoCool LLC (Albuquerque), not meeting the temperature needs for drug-delivery devices has a significant effect on patients and manufacturers. “Often sensitive products that require refrigeration during shipment are delivered at unacceptable temperatures. This creates several human factors problems in that patients cannot get the product on time, or worse yet, may use a product that has spoiled during shipment. Also, the cost of the spoiled drug can be very expensive for both the company and the patient.”
This poses a challenge for shipping, for which most manufacturers use ice packs and other cooling devices during transport. Because the ice packs add weight and therefore cost, freight can be a significant expense. Hyman is enthusiastic about the potential of the product. “Living in Texas, I wonder about the thermal conditions pharmaceuticals have been subjected to, and this packaging system could lead to more applications with thermal control.”
NanoCool LLC designed the packaging system to maintain a temperature of 2°–8°C for 48 hours. The system combines insulated packaging with a lid that uses evaporative-cooling technology. “NanoCool has developed a new shipping system with a goal of maintaining the required temperature during shipment, every time, so that the patient receives their medical product within temperature specification. Creating a new shipping solution to improve drug and medical shipments versus conventional shippers is our primary goal,” says Hoffman.
The system is shelf stable until needed for shipping, when the packager fills the box and presses a button on the lid to activate cooling. “I like that the activation is indicated very clearly with a blue logo disappearing,” says juror Privitera. The company can adjust sizes and other components based on customer needs.
Hoffman explains that the NanoCool system is up to 70% lighter and smaller than existing boxes and is environmentally friendly. He says the company specifically focused on environmental considerations during design. “NanoCool's packaging technology can ship a product utilizing a much smaller shipper than conventional-type polystyrene boxes and ice packs. Since the cooling technology is built into the insulation, our shipping systems are considerably smaller, easier to handle, and create less product to dispose of after the shipment, which has a positive environmental effect in that less material is sent to landfills.”
In addition, Hoffman says the system does not use polystyrene, which in many countries is considered an unfavorable material because of theories about its decomposition. “In Europe, disposing polystyrene-type products is believed to have a negative effect on the environment,” he explains.
The adsorption cooling technique is quite different from other technologies, and the company holds several patents for the process. And while consumer goods opportunities do exist, says Hoffman, NanoCool's focus is decidedly on medical and healthcare segments.
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like