Originally Published MDDI June 2001Note: This is the second part of a two-part article detailing the winners of the 2000 Medical Design Excellence Awards. If you haven't done so, you might like to read the first part of this article.In Vitro DiagnosticsGOLD WINNER
June 1, 2001
Originally Published MDDI June 2001
Note: This is the second part of a two-part article detailing the winners of the 2000 Medical Design Excellence Awards. If you haven't done so, you might like to read the first part of this article.
In Vitro Diagnostics
GOLD WINNER
BenchMark automated histology staining system
Submitted and manufactured by Ventana Medical Systems Inc. (Tucson, AZ)
 The BenchMark automated staining system is a modular device that fully automates the staining of tissue samples on glass microscope slides. It is the first histology instrument to automate the labor-intensive steps required in baking, dewaxing, cell conditioning, and staining tissue for the purpose of cancer and infectious-disease diagnosis, according to Ventana Medical Systems.
The BenchMark automated staining system is a modular device that fully automates the staining of tissue samples on glass microscope slides. It is the first histology instrument to automate the labor-intensive steps required in baking, dewaxing, cell conditioning, and staining tissue for the purpose of cancer and infectious-disease diagnosis, according to Ventana Medical Systems.
The instrument is used in the pathology department of hospitals and reference labs by laboratory technicians. The slides are processed with various reagents that remove embedded paraffin, pretreat tissue to expose targets, and bind and detect specific antigens or DNA and RNA sequences with a localized color change or fluorescence. These stained slides are then reviewed under a microscope by a pathologist to determine the diagnosis.
Developing the system required that a number of design challenges be overcome, says Peter Riefenhauser, Ventana's worldwide marketing manager. "The ability to individually control the temperature of each slide on a rotating carousel, and selectably provide seven bulk fluids to the slides was challenging," Riefenhauser states. "Tightly controlling and maintaining the liquid volume on each slide in a range from 25 ml to 700 ml was a challenge that not only involved the design of instrument hardware and control systems, but also included the formulation of reagents."
The BenchMark provides the ability to individually control the temperature of each slide on a rotating carousel, called the Thermoflex platform. This allows for flexibility in staining and run protocols. A creative technique for achieving very low residual slide volumes was developed in response to customer feedback. The Jet Wash or Jet Drain (patent pending) involves a stream of fluid directed at the edge of a slide that draws fluid off the slide without damaging or drying out the tissue, leaving a very low, evenly distributed volume.
To control evaporation, the BenchMark uses a patented liquid coverslip, forming a liquid shield that floats on top of the aqueous solution on the slide, and helping to control evaporation and temperature stability. To mix reagents with the aqueous volume on the slide, BenchMark uses patented vortex mixing, in which gentle, carefully positioned streams of air encourage mixing on the slide without physically touching or disturbing the patient sample.
The product design was intended to give users the ability to control multiple instruments of different types through a single user interface. Combined with bar code reading of patient samples and reagents, this interface enables the user to perform more-complicated multiple runs than was previously possible, according to the company.
The BenchMark "represents a major breakthrough in the application of design-for-manufacture principles," the company indicates. Manufacturing representatives were involved throughout the design process to give insight on areas of improvement. Great effort was put into component utilization from other Ventana instrument lines. Many of the assemblies are designed in such a way that they can only be put together one way to avoid assembly mistakes.
In order to further eliminate manufacturing errors and reduce tolerance stack-up issues, the majority of the BenchMark staining module is built up from a single, solid datum plane. Precision-machined posts extend from this common plane for accurate positioning and ease of assembly. The firmware built into the BenchMark instrument has self-test capabilities. This intelligence creates additional troubleshooting and diagnostic capabilities, helping the assembly and field technicians that work with the instrument.
This instrument line also marks the first product that Ventana completely developed in a virtual environment before building physical prototypes. Using state-of-the-art computer-aided engineering and electronic design automation tools, the product was modeled, analyzed, and modified multiple times before drawings for prototyping were generated. These powerful design tools provided the design team with the capability to test various design solutions and mathematically perform structural and heat-transfer analysis to compare different designs.
"The key achievement of this system is its ability to automate processes that were potentially hazardous, labor intensive, and inherently variable in their outcome," says Peter Riefenhauser, Ventana Medical Systems worldwide marketing manager. "There is no single design feature that provides this; rather it is the integration of the hardware, control, and reagent systems that achieves this," he adds. |
SILVER WINNER
Web-Enabled Drug Screening Helps Eliminate Errors
eScreen drugs-of-abuse testing system
Submitted and manufactured by eScreen Inc. (Overland Park, KS)
 The eScreen system is a Web-enabled workstation designed exclusively for drugs-of-abuse screening and data management. The system consists of a specimen cup and smart lid (eCup) and an optical Internet appliance (eReader) that scans, reads, and transmits results confidentially to a remote customer.
The eScreen system is a Web-enabled workstation designed exclusively for drugs-of-abuse screening and data management. The system consists of a specimen cup and smart lid (eCup) and an optical Internet appliance (eReader) that scans, reads, and transmits results confidentially to a remote customer.
The eScreen system is designed to remove the five barriers to point-of-care testing for drugs of abuse: aliquot sampling of a forensic specimen, timing and monitoring of assay, interpretation, transcription, and confidentiality. According to John Goodin, senior vice president of engineering and product development with eScreen Inc., "eScreen established a major design criteria that stipulated that the eCup was a 'dumb cup' accompanied by a 'smart lid'." He adds, "The development of the eReader and its optical image recognition software package (i.e., is it a line or isn't it a line?) evolved, over time, into a very sophisticated program."
According to the firm, the eCup is unique in the field of employment drug screening because the donor never comes into contact with the testing device. The cup is simply a specimen container that is used only for collecting the sample from the donor. The eCup smart lid contains the assay strips that perform the five-panel drugs-of-abuse screening function.
Says Goodin, "The primary achievement of the system is speed. Prior to the introduction of the eScreen cup and reader, the quickest turn-around time (under ideal conditions) for a laboratory based pre-employment drugs of abuse test was 36 hours—more often 48 hours. The eScreen system delivers the 95% negative component of pre-employment drug screen test results in one hour."
SUPPLIER FILE: Compression/Moll Industries Inc. (Lake Forest, CA)
Over-The-Counter and Self-Care Products
SILVER WINNER
Providing an Alternative to Self-Catheterization
TravelMate
Submitted and manufactured by Caring Hands Inc. (Hayden, ID)
 The TravelMate noninvasive female urinary device faced a number of challenges during its 18-month development cycle. "Experts in the CAD/mold-making profession said the design, as we wanted it, was not manufacturable. Five mold makers turned down the job as being too complex," explains Charles Robertson, principal designer of the device. "Eventually we found a talented team of mold makers who finished the mold, and they did a great job."
The TravelMate noninvasive female urinary device faced a number of challenges during its 18-month development cycle. "Experts in the CAD/mold-making profession said the design, as we wanted it, was not manufacturable. Five mold makers turned down the job as being too complex," explains Charles Robertson, principal designer of the device. "Eventually we found a talented team of mold makers who finished the mold, and they did a great job."
The device offers many of the advantages of external urinary catheters, without the problem of uncomfortable methods of attachment. Its noninvasive nature reduces the risk of urinary-tract infection.
The TravelMate consists of one piece of polyethylene that easily bends to conform to variations in the shape of the area surrounding the urethral orifice. The principal design challenge for this noninvasive device was to determine a way to achieve a comfortable leak-free seal between the device and the body while simultaneously keeping it small enough so that it could be easily positioned for use while sitting in a chair or standing with a minimal amount of undressing.
According to MDEA juror Krista Coleman, "The device provides a much-needed solution for women and will provide a definite benefit to many."
Says Robertson, "Our goal was to make a classic design that would be around for many years."He adds that "the project wouldn't have happened without some very talented people and the feedback from hundreds of prototype testers around the world."
SUPPLIER FILE: JB Engineering Inc. (Spokane, WA)
Radiological and Electromechanical Devices
GOLD WINNER
Seeking High Visibility
Echo-Coat ultrasound needles
Submitted and manufactured by STS Biopolymers Inc. (Henrietta, NY)
 This innovative product caught my eye because of the huge potential for improving the sampling precision for a wide variety of tissues. To provide the pathologists with the appropriate sample will undoubtedly increase the accuracy of diagnostics and decrease false-negative errors," says MDEA juror Krista Coleman.
This innovative product caught my eye because of the huge potential for improving the sampling precision for a wide variety of tissues. To provide the pathologists with the appropriate sample will undoubtedly increase the accuracy of diagnostics and decrease false-negative errors," says MDEA juror Krista Coleman.
Fellow juror Eliot S. Lazar adds, "the coating also strikes me as extraordinarily helpful to the end-user in order to locate a needle tip more readily when performing an ultrasonically guided procedure. Heretofore, needles have been difficult to identify, and with this coating the practitioner can more easily and adeptly carry out a successful procedure because of the ability to see the needle tip."
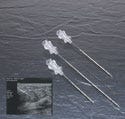
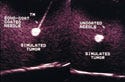 Echo-Coat ultrasound needles are coated to render the entire shaft highly visible during ultrasound imaging. The needles are used under ultrasound guidance to perform aspirations, biopsies, localizations, or amniocentesis procedures. Physicians in radiology private practice or in hospital settings use these needles to accurately obtain cytology and histopathology samples or to facilitate surgical procedures.
Echo-Coat ultrasound needles are coated to render the entire shaft highly visible during ultrasound imaging. The needles are used under ultrasound guidance to perform aspirations, biopsies, localizations, or amniocentesis procedures. Physicians in radiology private practice or in hospital settings use these needles to accurately obtain cytology and histopathology samples or to facilitate surgical procedures.
The coating is a layered polyurethane material that is applied to Type 304 stainless steel (i.e., a needle cannula). It has a porous microstructure that entraps microbubbles of air to enhance the echogenicity regardless of the needle angle relative to the ultrasound beam.
Under ultrasound imaging, a standard needle must be held perpendicular to the ultrasound beam to be visible. At nonorthoganol angles, the sound waves are reflected off the needle shaft at angles that do not reach the transducer face. As a result, the needle is not visible. With Echo-Coat ultrasound coating, the trapped microbubbles of air reflect sound waves back to the transducer—even when the needle is at a very steep angle in relation to the transducer. These reflections enable the needle to be clearly visible along its entire shaft.
New applications for the coating technology are continuing to be developed, says Susan Stalls, STS Biopolymers product manager. She explains, "We started with fine needles for aspiration and recently added breast localization needles. We plan to add core biopsy needles later in the summer and then expand further in the fall."
According to Susan Stalls, product manager for STS Biopolymers, developing Echo-Coat ultrasound needles required "almost 10 years from concept to market. In part, this was because it was done entirely by in-house research and development." Stalls adds, "We approached the lack of visibility of medical devices under ultrasound from a totally different angle. Historically, people had tried to change the surface of the metal by dimpling or roughening it. We approached it from 'what is the most echogenic biocompatible material?' Air is great. Now how do we get air onto a device—that's where our coating expertise married our knowledge of ultrasound contract agents, and we began to develop a coating to go over the needle surface to accomplish echogenicity." |
SILVER WINNERS
Listening to Customers Provides Key to Improved Device Design
DirectView CR800 system
Submitted and manufactured by Eastman Kodak Co. (Rochester, NY)
 The Kodak DirectView CR800 System is a computed radiography medical x-ray image-capture and image-processing system that uses current x-ray exam-room exposure sources and cassette-based techniques while delivering the advantages of digital technology. With an easy-to-use graphical user interface and high image quality, the DirectView CR800 system facilitates wide electronic distribution of x-ray images to radiologists responsible for diagnostic analysis, to referring physicians for review in preparation for treatments and therapies, and to electronic archives where images can be stored for future reference.
The Kodak DirectView CR800 System is a computed radiography medical x-ray image-capture and image-processing system that uses current x-ray exam-room exposure sources and cassette-based techniques while delivering the advantages of digital technology. With an easy-to-use graphical user interface and high image quality, the DirectView CR800 system facilitates wide electronic distribution of x-ray images to radiologists responsible for diagnostic analysis, to referring physicians for review in preparation for treatments and therapies, and to electronic archives where images can be stored for future reference.
Typical imaging applications include chest x-rays for the detection of heart and lung diseases, bone x-rays for fractured bones or bony tumors, and soft-tissue images such as the kidneys and gastrointestinal system. The DirectView CR800 system, in conjunction with the Kodak DirectView remote operations panel, is designed to allow multiple user stations to be placed throughout the imaging environment, resulting in improved workflow, enhanced patient care, and reduced costs.
Dennis E. Sneddon of Kodak explains that reliability and maintaining a small footprint were primary concerns of the development team. "High reliability was addressed by using modeling and continuous reliability testing of critical subsystems during the design process. The subsystem testing identified issues prior to the final designs, and provided an opportunity to address and resolve issues prior to manufacturing."
Sneddon adds that internal Kodak shops played a key role in the concept, design and production phases, and early reliability testing and verification allowed the team to make changes as needed during the process to improve the various subsystems. He explains that "the device's footprint, the customer interface, and workflow were verified by 'voice of customer' processes and user contacts."
Says Sneddon, "The company's industrial design department was instrumental in quickly turning concepts into prototypes, and prototypes into finished equipment mock-ups. Prototyping played a key role in the speed at which design variations were tested and evaluated."
Throughout the project to design and develop the DirectView CR800 system, the engineering team listened carefully to its customers. Hospitals, clinics, and medical imaging centers were looking for new alternatives to digitally acquire radiographic images that would improve x-ray services, increase productivity, reduce costs, and ultimately position their operations favorably against increased competition. Likewise, radiologists and radiologic technologists were seeking ways to enhance workflow, increase patient throughput, improve output quality, and facilitate better overall image management.
One of the challenges Kodak's engineering team faced in developing the system was that of producing a digital high-quality image-capture system that could enable decentralized placement of devices within medical diagnostic imaging areas. Usability scenarios that defined the physical and functional requirements for the system and the user interface were key to the effort. By thoroughly defining parameters in advance, the Kodak design team was able to effectively develop and deliver a fully tooled and tested product within the constraints and boundaries of an extraordinarily aggressive project timeline.
SUPPLIER FILE: Elcan Optical Technologies, a Raytheon corporation (Midland, ON, Canada), Mercury Aircraft (Hammondsport, NY), Gillette Machine and Tool Company Inc. (Rochester, NY), Solectron Technology Inc. (Charlotte, NC), IBM Corp. (Rochester, NY), Micro Industries (Centerville, OH), Kaman Industrial Technologies Corp. (Rochester, NY), Thompson Industries (Port Washington, NY), AJL Manufacturing Inc. (Rochester, NY), Badge Machine Products Inc. (Canandaigua, NY), K&H Precision Products (Honeoye Falls, NY), Noma Appliance and Electric (Tillsonburg, ON, Canada), Delta Electronics Inc. (Research Triangle Park, NC)
According to Kodak's Dennis E. Sneddon, "Our design team had several overarching goals as we designed the CR 800 including: The need for high reliability, small footprint (everything in one box), and design of a customer interface that focused on enhancing workflow." |
Using Vascular Brachytherapy to Prevent Restenosis Following Angioplasty
Beta-Cath
Submitted and manufactured by Novoste Corp. (Norcross, GA)
 In-stent restenosis is a common problem associated with angioplasty procedures, and often requires coronary-artery-bypass surgery. The Novoste Beta-Cath system can be used by the interventional cardiologist and radiation oncologist in the cardiac catheterization laboratory, and is designed to deliver a prescribed dose of beta radiation to the coronary artery vessel wall.
In-stent restenosis is a common problem associated with angioplasty procedures, and often requires coronary-artery-bypass surgery. The Novoste Beta-Cath system can be used by the interventional cardiologist and radiation oncologist in the cardiac catheterization laboratory, and is designed to deliver a prescribed dose of beta radiation to the coronary artery vessel wall.
Tom Weldon, chairman of Novoste, notes that the Beta-Cath design had to overcome two challenges. "One is, it's basically a hydraulic system. Because the device had some electrical components, it presented a real design challenge to keep the fluid hydraulic systems separate from the electrical systems in a handheld device that would operate reliably. An additional challenge was developing the appropriate isotope in a sealed source container in a size with the level of intensity necessary to provide the therapeutic dose targets that we had for the device."
Weldon says the device is distinguished by its novel use of hydraulics "and the associated use of a seed train, which is literally like a bunch of cars parked one end to the other." He suggests that isotope selection for the device provides an additional advantage. "We chose an isotope a with a very long half-life— strontium-90—so the seed train can be reused many, many times, spreading the device cost over hundreds of patients."
SUPPLIER FILE: The Innovation Factory (Norcross, GA), Plexus (Bothell, WA), BeBig (Berlin), and Colorado MedTech (Boulder, CO)
Rehabilitation and Assistive-Technology Products
GOLD WINNER
Prototype Evaluation Offers Key to Prosthesis Development
Pathfinder prosthetic foot
Submitted and manufactured by Ohio Willow Wood Co. (Mt. Sterling, OH)
 Among the principal challenges faced by the developers of the Pathfinder prosthetic foot was the need to simulate human gait with natural ankle motion, says Jeff Doddroe of Ohio Willow Wood. The award-winning version of the device included a number of significant engineering and design accomplishments. Doddroe explains that "composite materials used in the toe springs and the foot plate needed to have increased flexibility . . . without sacrificing strength."
Among the principal challenges faced by the developers of the Pathfinder prosthetic foot was the need to simulate human gait with natural ankle motion, says Jeff Doddroe of Ohio Willow Wood. The award-winning version of the device included a number of significant engineering and design accomplishments. Doddroe explains that "composite materials used in the toe springs and the foot plate needed to have increased flexibility . . . without sacrificing strength."
Another advantage, Doddroe adds, is the use of adjustability features that enable the unit to be customized for a wide range of users. These features include an adjustable heel, anterior and posterior slides, rotation adjustment, and five toe spring resistances.
According to MDEA juror Michael Wiklund, among the winning qualities of the Pathfinder is the fact that it not only simulates normal gait, but provides a degree of versatility to the user. He states, "When you consider the nuances of designing an artifical foot that will function effectively in many different use scenarios, ranging from climbing stairs to running to standing in place, it emerges as quite an accomplishment. It is even more significant when when you consider that companies have been trying to design good ones for decades—centuries, really. When you consider how much the product will contribute to the quality of life for the recipient, you have the makings of a truly deserving award winner."
MDEA juror Krista Coleman adds, "The improvement over previous designs for the foot and ankle is incredible. They managed to improve several areas that have been problematic for patients and prosthetists." She adds, "Another advantage is the conservation of energy for the user owing to the capability to temporarily store some energy in the springs during early loading of the prosthetic device, and to return that energy to the user later during the phase of gait where the toes are pushing the body forward."
The Pathfinder prosthetic foot can be used by lower- extremity amputees who have moderate to high activity level, a body weight of less than 250 lb, a foot size ranging from 23 to 31 cm, and 9 in. of clearance from the distal end of their limb to the floor. The device's shock absorption, energy-storing capacity, and unique ankle motion work together to provide a greater range of controlled motion to the user, resulting in greater comfort, less fatigue, and improved balance. The Pathfinder is designed for use in the normal environment of an active lower-extremity amputee.
The current generation of the prosthesis evolved through a lengthy evaluation of several prototypes. Doddroe explains, "The most significant contribution made outside [the company] was by the amputees involved in the field testing of the Pathfinder." The objective was to develop a product that would offer improved energy storage and return, shock absorption, increased stability, range of motion, and a smooth transition from heel strike to toe off. The result was a triangular design, a closed shape with three main sides.
Conventional prosthetic feet are based on a cantilever design with a single side and a single joint. The three sides of the Pathfinder triangle—the pneumatic heel spring, the composite toe springs, and the composite foot plate—are connected at three main joints: the toe connectors, the heel connector, and the proximal connector. The triangular concept of the Pathfinder is considered to be advantageous because each side can function as an independent energy-storing component. The connecting joints at both ends limit the deflection of each side, resulting in greater flexibility than a cantilevered design, the firm indicates. Increasing the flexibility of the elastic components enhances their ability to store energy, producing a greater dynamic response. The Pathfinder's triangular design enables each side to act synergistically with the others to provide an optimal gait.
The product design also provides a "polycentric ankle motion" that enables the device to exhibit a complex motion "that users claim to be much like a natural ankle," according to Ohio Willow Wood. The result is that amputees experience smoother foot motion and a more natural feel. The foot is also more stable and provides better balance for amputees, the company adds.
According to the manufacturer, the Pathfinder enabled the company to enter a segment of the prosthesis market where it had not previously been a competitor. There are also several production features of the Pathfinder that can be incoporated into existing product lines at Ohio Willow Wood, which will enhance performance of those products. This design also reaffirms the company's commitment to providing new and improved technology in the prosthetics industry and offering freedom of motion to amputees.
Approval and coding from several governing bodies within the orthotic and prosthetic industry is still pending. While the company has had several independent Veterans Administration (VA) offices approve individual purchases of the Pathfinder by veterans, it is still awaiting final approval by the VA. The Pathfinder has passed all applicable ISO structural tests. Doddroe notes that the company intends to enhance the next generation of Pathfinder to increase its weight limit from 250 to 350 lb.
SILVER WINNERS
Hearing Aid Uses Digital Perception Processing
Claro and WatchPilot digital hearing instruments
Submitted and manufactured by Phonak (Warrenville, IL)
 Hearing aid development has already attained two different goals: devices have been made smaller, and communication in background noise has been improved. "Today we want to make it easier to hear and understand speech in quiet places, noisy places, and anywhere the wearer wants to hear," says Laura Voll of Phonak AG. "We also want the sound quality to be as natural as possible and control over the hearing aid as convenient as possible."
Hearing aid development has already attained two different goals: devices have been made smaller, and communication in background noise has been improved. "Today we want to make it easier to hear and understand speech in quiet places, noisy places, and anywhere the wearer wants to hear," says Laura Voll of Phonak AG. "We also want the sound quality to be as natural as possible and control over the hearing aid as convenient as possible."
Claro is a digital hearing instrument that can be used in all environments. Claro uses two unique technologies for reducing background noise. The WatchPilot is a first-of-its kind remote control contained in a wristwatch, according to Phonak.
One of the technologies used in Claro to reduce background noise is adaptive digital AudioZoom. AudioZoom is the first hearing-aid system that uses two microphones to create a directional response. Conversation from the front is increased, while sounds from the sides and back (background noise) are effectively reduced.
Claro employs digital perception processing, a method that uses 20 overlapping and interdependent channels to process sound just like the normal human ear. Contained in its microprocessor is a computer model of the normal ear.
The biggest challenge for any hearing instrument is to effectively reduce background noise. Claro uses two unique technologies to do this, fine-scale noise cancellation (FNC) and adaptive digital AudioZoom (dAZ). FNC scans the signal in each channel and identifies speech relative to steady-state noises. It increases the speech signal and reduces the noise as much as possible. The manufacturer states that the dAZ uses a two-microphone array system to reduce the sound coming from other directions and improve reception of conversational speech.
Says Voll, "There have already been enhancements in the system." She explains, "One of the advantages of offering digital signal processing that is controlled using a personal computer is that upgrades occur through software, rather than hardware."
Simplifying Personal Oxygen Use
HELiOS personal oxygen system
Submitted and manufactured by Tyco Healthcare Puritan-Bennett (Indianapolis)
 HELiOS, which stands for high-efficiency liquid-oxygen system, is designed to provide chronic obstructive pulmonary disease patients with their prescribed oxygen in a home-care environment. The system consists of a reservoir and a portable unit. The reservoir holds up to 46 L of liquid oxygen, and is used to fill the portable unit for ambulatory or mobile use. The portable unit includes an innovative pneumatic 4:1 conserver device, "which allows for the best combination of duration, small size, and light weight—3.5 lb when full," according to the manufacturer. Together, the two units provide a home oxygen system "that can effectively be 'worn' rather than 'lugged around,' which promotes patient mobility and allows for better patient prescription compliance," say Lee S. Toma, senior engineer with Puritan Bennett.
HELiOS, which stands for high-efficiency liquid-oxygen system, is designed to provide chronic obstructive pulmonary disease patients with their prescribed oxygen in a home-care environment. The system consists of a reservoir and a portable unit. The reservoir holds up to 46 L of liquid oxygen, and is used to fill the portable unit for ambulatory or mobile use. The portable unit includes an innovative pneumatic 4:1 conserver device, "which allows for the best combination of duration, small size, and light weight—3.5 lb when full," according to the manufacturer. Together, the two units provide a home oxygen system "that can effectively be 'worn' rather than 'lugged around,' which promotes patient mobility and allows for better patient prescription compliance," say Lee S. Toma, senior engineer with Puritan Bennett.
Toma notes that the system's biggest achievement was creation of the oxygen conserver. "Powered by the pressure of the oxygen gas," he explains, "it eliminates the need for a heavy and unreliable battery. Based loosely on our previous design, which only delivered oxygen on inhalation and saved about 50% over a standard continuous flow prescription, the new design saves about 75% by using a new algorithm evaluated in clinical tests." Says Toma, "This allows a very small unit to hold enough liquid to last up to ten hours at the most common 2 liter-per-minute setting. It also allows the homecare provider to save time and money on liquid deliveries."
"The main development challenge of the project was to create a system that would appeal to users based on ergonomics and style, while also making the system economically viable for the homecare providers," says Lee S. Toma. |
SUPPLIERS FILE: Omnica Corp. (Irvine, CA)
Surgical Equipment, Instruments, and Supplies
Light-Activated System Assists Rapid Sealing of Sutures
Tissuebond applicator and Tissuemed 180 light source system
Submitted by DCA Design Consultants (Warwick, UK); manufactured by Tissuemed Ltd. (Leeds, UK)
 The Tissuebond system consists of two main components, a custom-designed applicator and a light source for activating the Tissuebond sealant. The disposable applicator is similar in size and shape to a pen and contains the Tissuebond sealant. Composed of porcine albumin, glycerol, and water for injection, the sealant also contains methylene blue to give the sealant a distinctive blue color. This enables the surgeon to see exactly where the sealant has been applied. It also acts as the chromophore, or switch, which activates the bonding process when a special light is applied to the Tissuebond material.
The Tissuebond system consists of two main components, a custom-designed applicator and a light source for activating the Tissuebond sealant. The disposable applicator is similar in size and shape to a pen and contains the Tissuebond sealant. Composed of porcine albumin, glycerol, and water for injection, the sealant also contains methylene blue to give the sealant a distinctive blue color. This enables the surgeon to see exactly where the sealant has been applied. It also acts as the chromophore, or switch, which activates the bonding process when a special light is applied to the Tissuebond material.
The main applications of Tissuebond are to act as a tissue sealant and to aid anastomosis. The single-use Tissuebond applicator is designed to deliver a precise amount of sealant during surgical procedures. A finger-controlled lever on the applicator enables the user to dispense an accurately controlled bead of adhesive onto the area of tissue requiring bonding. Tissuebond will be used in surgical procedures where blood loss and time to obtain clotting should be kept to a minimum. Often this will be in cardiovascular surgery procedures where surgeons have to join two ends of blood vessels together to bypass blockages in the vessel. It will also be used to join human vessels with manufactured vessel-grafting materials such as Dacron and PTFE.
According to DCA Design Consultants, "The design process for the applicator involved a detailed study of surgeons' needs and current practices. Then, having identified a range of design concepts, DCA constructed working models and rigs to determine the optimum ergonomic solution. DCA also liaised closely with a specialist mold maker to optimize the design of the components to suit high-volume manufacture." The design firm adds, "In complete contrast to the applicator, the lightsource is designed for low-volume manufacture and therefore uses processes such as RIM casting, CNC machining, and metal fabrications using laser-profiled blanks."
The Tissuemed 180 light source system delivers a cold, filtered light beam through a handheld wand that is directed at the area where Tissuebond has been applied. The surgeon controls the beam by pressing a foot switch. The methylene blue in Tissuebond absorbs light emitted from the system. Within a few seconds of applying the light source to the Tissuebond, an obvious change of color from blue to clear or light blue gives the surgeon visual confirmation that the sealant has been successfully activated. The light source is used during surgical operations to cure the tissue adhesive at the site of the wound. It is typically used in an operating theater environment. All the components that may contact the patient directly or indirectly are capable of being sterilized.
Tissuebond requires no preparation and can be used directly out of the refrigerator, allowing the surgeon to react to bleeding sites immediately. The rapid sealing of the sutured area forms a strong yet flexible barrier, allowing the natural healing process to begin.
Says DCA, "Tissuemed looked at their own in-house skills and realized that the skills required were outside their own areas of expertise. Secondly, time to market was important and therefore the use of an external agency with large resources and efficient working practices was required." |
SILVER WINNERS
Developing a Noninvasive CABG System
Aortic connector system
Submitted by redgroup (Minneapolis); manufactured by St. Jude Medical (St. Paul, MN)
 The St. Jude aortic connector system delivers an implanted device designed to create the proximal anastomosis of an aortic autologous vein graft. It is intended to be used as a noninvasive means to cardiac bypass surgery and eliminate the need for hand sewing. Surgeons are the intended primary users.
The St. Jude aortic connector system delivers an implanted device designed to create the proximal anastomosis of an aortic autologous vein graft. It is intended to be used as a noninvasive means to cardiac bypass surgery and eliminate the need for hand sewing. Surgeons are the intended primary users.
The implantable device is made of nitinol. The delivery device is constructed of injection-molded ABS, and the release tubes are made of stainless steel.
The primary design problem addressed was loading the system with a vein, then delivering and connecting it successfully to the aorta with accuracy and limited hand movement. The overall shape and geometry of both the aortic cutter and the delivery device were specific to each task they had to perform. Based on user research, the aortic cutter needed to be used both in a syringe and pipette configuration. The aortic delivery device has a pipette configuration, allowing for greater accuracy and reduced hand movement. The button guard was specifically designed to protect the button from accidental deployment, yet allow the surgeon to access the button with minimal finger movement.
According to Lars Runquist, a principal in redgroup, there were several key relationships that were useful in the development process. "The molding vendor played a key role," says Runquist, "helping in tool development in parallel with the design of the product. The result was very few tooling revisions, and a faster program."
Runquist explains that succesfully addressing ergonomic considerations was a key achievement of the design—"how the surgeon handles the device, including both the cutter and the applicator." He adds that efforts to enhance the device are ongoing. "We have already completed additional size variations of the product," he states.
Creating a New Point of View for Surgeons
InsideView
Submitted and manufactured by LSI Solutions (Rochester, NY)
 The InsideView medical display system, manufactured by LSI Solutions, uses advanced video-projection technology to display a high-resolution video image on a sterile, disposable screen appropriately positioned within the sterile surgical or interventional procedure field.
The InsideView medical display system, manufactured by LSI Solutions, uses advanced video-projection technology to display a high-resolution video image on a sterile, disposable screen appropriately positioned within the sterile surgical or interventional procedure field.
"This is a vast technical improvement for practitioners in that they will not need to divert their attention from the procedure to turn and view a screen in some more-distant location," says MDEA juror Eliot S. Lazar. "The benefits are to both the end-user and the patient."
Physicians using this unique visualization technology can operate in a natural and comfortable way because they can simultaneously view the internal operative site and their hands. Communication and teaching are improved during procedures by allowing operators to point to and touch the video image.
According to LSI Solutions, the InsideView medical display system "utilizes advanced video projection technology to display a high-resolution video image on a sterile screen appropriately positioned within the sterile surgical or interventional procedure field." The firm adds, "Physicians using this unique visualization technology can once again operate in a natural and more comfortable way because they can simultaneously view the internal operative site and their hands."
The system also supports communication and teaching efforts during procedures by allowing operators to point to and touch the video image.
LSI suggests that most videoscopic medical procedures can benefit from the system. Example application areas include general surgery and urologic, gynecologic, and cardiac interventions. Endoscopy is another potentially enormous market. Radiologic procedures, especially fluoroscopic procedures for diagnostic and therapeutic interventions, will benefit from application of this technology, the company indicates.
The system video projector is held within a proprietary optical fixture mechanism manufactured by LSI. The EtO-sterilized screen kits are made of high-grade, lightweight optical plastics that are conveniently packaged for cost-effective, single-patient use.
Making the Prospect of a Breast Biopsy Less Intimidating
Mammotome handheld breast biopsy system
Submitted by Plexus Corp. (Neenah, WI) and Herbst LaZar Bell (Chicago); manufactured by Ethicon Endo-Surgery (Cincinnati)
 With the incidence of breast cancer on the rise among women of all ages, it has become increasingly important to develop devices that facilitate breast biopsy procedures, explains Cassie McQueeny-Tankard of Herbst LaZar Bell Inc. (HLB). The Mammotome handheld breast biopsy system is a minimally invasive, highly accurate, mobile instrument for helping doctors diagnose breast cancer at the earliest stages while increasing comfort to patients.
With the incidence of breast cancer on the rise among women of all ages, it has become increasingly important to develop devices that facilitate breast biopsy procedures, explains Cassie McQueeny-Tankard of Herbst LaZar Bell Inc. (HLB). The Mammotome handheld breast biopsy system is a minimally invasive, highly accurate, mobile instrument for helping doctors diagnose breast cancer at the earliest stages while increasing comfort to patients.
The new Mammotome system creates a less-intimidating and more-comfortable arrangement for the patient with minimal procedure preparation time. The procedure may be completed in less than an hour in a doctor's office or on an outpatient basis under a local anesthetic and requires no surgery or stitches. It allows the patient to lie comfortably on her back.
The Mammotome probe is inserted only once into the patient's breast via a small incision. Once inserted and positioned using ultrasonic imaging, the needlelike probe can collect multiple samples by means of vacuum aspiration and an internal rotating cutter. The vacuum draws the sample into the probe aperture within reach of the cutter. From there, tissue samples can be obtained in and around the targeted area. Even though the incision is smaller, these samples can be eight times the weight of samples obtained with traditional spring-loaded biopsy equipment.
According to McQueeny-Tankard, "HLB contributed to the electrical engineering of the onboard microprocessor, which automates the sampling process. Biopsy sampling is no longer manual." She adds that the use of a positioning sensor enables the color touch-screen monitor to reflect the exact position of the cutting tip. "An easy-to-follow graphical user interface was developed to give surgeons maximum control over the location from which the biopsy sample is taken," she states.
The engineering design team created a two-motor cutting drive system self-contained in the base unit and connected to a lightweight handpiece that eliminated the need to table mount the cutter assembly. Lightweight, flexible cables connect the base to the disposable handpiece, which is lighter and easier to control than its predecessor. This handheld unit, which incorporates cutter position sensors, allows physicians to place the sampling probe accurately and obtain larger samples of suspect tissue.
"The cutting drive system was also redesigned to include the new Smart Cutter, a unique feature that provides direct feedback control of both cutter translational and rotational speeds," says McQueeny-Tankard. "When either the translational speed or rotational speed is not at the desired rate due to increased or decreased loading on the system, the control feature modifies the power to the motor. This allows the speeds to remain near their desired levels."
In addition, the handheld cutting probe was ergonomically designed to allow for easy manipulation and procedure control through the soft-touch finger-control keypad. Precise position control lets the cutter close the aperture through which the sample enters without bottoming out at the end of the probe. Good position control—within 0.001 in.—enabled the design team to minimize the length of the cushion, or "dead zone," at the end of the probe. Incision of the tissue and completion of the sampling as directed is achieved without causing damage to healthy surrounding tissue.
The technology at the heart of the Mammotome system is the SmartVac computer-controlled vacuum system. According to McQueeny-Tankard, this enables the vacuum to cycle on and off, and optimizes the vacuum in accord with the cutter activity. The vacuum retrieval system allows the caregiver to take multiple samples of a lesion while the needle probe remains in the breast. This second-generation device is able to obtain larger samples than its predecessor. "As a result, there are fewer dry taps, or the inability to obtain an adequately sized sample of the suspect tissue," McQueeny-Tankard indicates.
SUPPLIER FILE: Phillips Plastics (Hudson, WI), MPE Inc. (Milwaukee, WI), Marksman Metals (St. Michael, MN), Innovative Machining (Neenah, WI), Identco (Ingleside, IL), Scotts Models Inc. (Cincinnati), MPI International Inc. (Rochester Hills, MI), Arrk (San Diego), Cut Craft Inc. (Ft. Worth, TX)
Safer Medical Waste Handling
Neptune waste-management systemSubmitted and designed by American Immuno Tech Inc. (AIT; Costa Mesa, CA); manufactured by Bio-Medical Devices Inc. (Costa Mesa, CA) for Stryker Instruments, a division of Stryker Corp. (Kalamazoo, MI)
 Management and disposal of fluid and certain airborne medical wastes has posed difficulties for hospitals. The Neptune waste-management system was developed to provide viable waste management alternatives. The system is intended to be used in the operating room, pathology and surgical centers, emergency rooms, and doctors' offices to evacuate, collect, and dispose of hazardous surgical fluid waste as well as to evacuate smoke generated from electrocautery or laser devices.
Management and disposal of fluid and certain airborne medical wastes has posed difficulties for hospitals. The Neptune waste-management system was developed to provide viable waste management alternatives. The system is intended to be used in the operating room, pathology and surgical centers, emergency rooms, and doctors' offices to evacuate, collect, and dispose of hazardous surgical fluid waste as well as to evacuate smoke generated from electrocautery or laser devices.
Says David H. Mills, AIT director of business development, "The technical challenge of moving bloody debris through the system without a clog building up and compromising the surgeon's suction was the biggest obstacle. Secondly, delivering high quality suction without creating excessive noise was also a challenge for the engineering team."
The system consists of two components—a rover and a docking station. The rover is designed to be located in the surgical suite or where the fluid waste or smoke is generated. The docking station is used to collect fluid waste from the rover unit prior to disposal. The device can collect as much as 20 L of fluid at one time, with fluid volume measured electronically, and is accurate to approximately 250 ml. This feature delivers a safer means of monitoring fluid absorption during high-fluid procedures, including transurethral radial prostatectomy or endometrial ablation.
Unique to the Neptune system is the incorporation of multiple products into one common footprint, says Mills. The system combines a traditional suction canister-tree with that of a smoke evacuator, and adds an optional power IV pole to aid in the delivery of irrigation fluid during high-fluid procedures. Equipped with the ability to accurately track the fluid waste collected via a digital display, the rover aids the gynecological surgeon in monitoring fluid absorption.
Copyright ©2001 Medical Device & Diagnostic Industry
You May Also Like


