Measuring and mitigating the risk of extractables poses a significant challenge in the medical device field.
CHEMICAL CHARACTERIZATION
|
Illustration by iSTOCKPHOTO |
One of the greatest challenges in medical device chemical characterization is performing adequate assessment of biological or toxicological risks from extractables that can compromise patient safety. EN/ISO 10993-17 has clearly stated that risk assessments are a part of material biocompatibility and that they are necessary for the assurance of biological safety.1 Toxicological hazard of the chemical constituents of the materials from which a medical device is made should be considered in biological safety. Therefore, the first step for a biological safety assessment is to characterize the chemicals of the materials. Toxicological hazards can be identified by understanding the toxicity of materials or extracted chemicals.
A systematic analysis of biological risks can be found in the general principles set out in clause 3 of ISO 10993-1.2 Do not be tempted to use the matrix in ISO 10993-1 as a checklist to perform a standard set of tests. Instead, use the principles to develop an appropriate scientific evaluation program based on the specifics of the device.
The results of all tests should be interpreted in the context of the overall risk assessment to determine whether a specific outcome indicates acceptable risk. Such a collaborative approach emphasizes the need for an overall scientifically valid risk assessment. The manufacturer, the analytical chemists, and the toxicological risk assessor must have input and each must be a significant contributor to the assessment process. A common question asked is: Does assessment of the risk that may be posed by a particular material or combination of materials require chemical characterization? This article addresses chemical characterization and defines the role of toxicological risk assessment for medical devices.
Background
Risk assessment has only recently been integrated into international standards and endorsed as an integral part of chemical characterization and biocompatibility studies for medical devices. The suitability of a medical device for a particular use involves balancing any identified risks with the clinical benefit to the patient associated with its use.
EN/ISO 10993-17 states that “among the risks to be considered are those arising from exposure to leachable substances arising from medical devices.” The standard provides a method for calculating maximum tolerable levels that may be used by “other standards-developing organizations, government agencies, and regulatory bodies. Manufacturers and processors may use the allowable limits derived to optimize processes and aid in the choice of materials in order to protect patient health.”
Risk assessment, as explained in EN/ISO 10993-17, is really a tool that has evolved to enable decision making. Manufacturers and processors may use derived allowable limits to aid in choosing the most appropriate material for a particular medical device application.
Toxicological risk assessments have a long history with strong ties to Europe (BS 5736 series standards), FDA, Environmental Protection Agency (EPA), and the Occupational Safety and Health Administration (OSHA). Now ISO 10993 standards for medical devices prescribe the use of toxicological risk assessments for biological studies, including materials characterization and degradation studies. To be effective, the risk assessment must be well organized, documented, and evidence-based for use in support of decision making with respect to product or material safety.
Toxicological Hazard: A property of the chemical constituents of the materials from which a medical device is made and chemical composition should be considered in relation to hazard identification. Risk Characterization: The final stage in the risk assessment process and involves predicting the frequency and severity of effects in exposed populations. Chemical Category: A group of chemicals whose physicochemical and toxicological properties are likely to be similar or follow a regular pattern as a result of structural similarity. |
The aim of the assessment should be to identify any biological hazards inherent in the materials used in the medical device and to estimate the risks resulting from hazards for the intended use. The goal is to develop a process that ultimately protects public health and establishes the safety of medical devices. The objective is supported by EN/ISO 10993-17 in subclause 4.3 of the general principles for establishing allowable limits, which states that “the safety of medical devices requires an absence of unacceptable health risk.”
A medical device manufacturer is responsible for ensuring its devices' biological safety and for documenting the assessment of toxicological risks and establishing the effectiveness of the analysis. Evidence must be provided that an appropriate toxicological risk assessment has been carried out so that the OEM can ensure that public health is not endangered.
EN/ISO 10993-17 also adds that “where risks associated with exposure to particular leachable substances are unacceptable, this part of ISO 10993 can be used to qualify alternative materials or processes.” This is another example of the way risk assessment can be used as a mechanism for critical decision processes.
Additional information from biocompatibility tests or on prior use of the materials may be used to provide a basis for further assessment of risks. Acceptable results from appropriate biological tests, such as those listed in the EN/ISO 10993 series of standards, may give a degree of assurance that the risk of adverse reactions occurring during clinical use is low. These tests differ from classical toxicity tests in that they typically attempt to mimic the conditions of clinical exposure to medical devices.
Standardized toxicological tests are amenable to the generation and comparison of data from a wide range of test materials within or across chemical platforms. Because standardized protocols must be broadly applicable for the study of a variety of different materials, they cannot realistically be expected at the same time to address highly focused mechanistic toxicological issues associated with only one or a few chemical compounds.3 This point of view is also expressed in an updated UK competent authority guidance note from 5 EC Medical Devices Directive, published January 2006.4 The Guidance on the Biological Safety Assessment states that
These tests, commonly termed biocompatibility tests, differ from basic toxicity tests in that they typically attempt to mimic the conditions of clinical exposure to medical devices and thus provide an indication of the probability of adverse effects arising during use. They tend, as a result, to be less sensitive than basic toxicity tests and are thus a less discriminating indicator of risk. Biocompatibility test data should therefore be used to complement an assessment based on materials characterization, rather than as a replacement for it.
Toxicological hazard is a property of the chemical constituents of the materials from which a medical device is made, and chemical composition should be considered in relation to hazard identification. If significant risks arising from hazardous residues are identified by chemical characterization, their acceptance should be assessed in line with established toxicological principles. Biocompatibility tests identified in EN/ISO 10993 may be used to provide further assessment of risk.
Components of Risk Assessment
EN/ISO 10993-17 is an ambitious, much needed guidance document that defines and documents consistent practices for evaluation of the risk factors for specific leachable substances. The probability that an adverse effect will arise from exposure to a chemical depends on its inherent toxicity, but also on the amount to which a subject is exposed and the route of that exposure.
EN/ISO 10993-17 provides a systematic method for assessing complex solutions or extracts. The standard uses four basic steps that are commonly used in the risk assessment process. These steps, defined by the National Academy of Sciences (NAS), are as follows:5
Hazard identification.
Dose-response assessment.
Exposure assessment.
Risk characterization.
These four steps, when accurately defined and evaluated, result in a statistically derived probability that an adverse effect will occur at a defined exposure level. Risk characterization is the process in which the dose-response assessment and exposure assessments are integrated to predict risk to specific populations. Risk characterization is the final stage in the risk assessment process and involves predicting the frequency and severity of effects in exposed populations.
To establish a tolerable intake (TI) for a specific leachable substance, modifying factors are applied to the data for noncancer endpoints so that an appropriate intake value can be established. For example, the modifying factor is derived as the product of various component uncertainty factors.
One example of a commonly used uncertainty factor is the factor used in extrapolating the effects of animal studies to humans. If only limited long-term exposure studies were available, a higher uncertainty factor leading to a lower acceptable exposure in the human population would be employed. It is noted in the standard that when this factor is combined with other uncertainty factors, modifying factors may be expected to differ by two orders of magnitude. Uncertainty factors and ultimately the modifying factors are derived on a case-by-case basis. They are highly dependent on the quality of the toxicological database.
|
Figure 1. (click to enlarge) This graph demonstrates the quantitative relationship between the level of exposure and the intensity or occurrence of a resulting adverse health effect. Dose determines the biological response. |
An important step in any estimation of chemical toxicity is generating a dose-response curve, a graphic representation of the quantitative relationship between the level of exposure and the intensity or occurrence of a resulting adverse health effect. Figure 1 shows a typical dose-response relationship. A dose or concentration of a chemical substance that does not produce any adverse effect—i.e., no observed adverse effect level (NOAEL)—is identified, usually from toxicological studies involving animals, but sometimes from epidemiological studies of human populations. A modifying factor is applied to the NOAEL to derive a tolerable daily intake (TDI). TDI is the intake or concentration to which it is believed a person can be exposed daily over a lifetime without deleterious effect.
Manufacturing, assembling, packaging, and sterilization of medical devices tend to result in a multiplicity of process chemicals that can potentially migrate into surrounding tissues and body fluids. Many of these chemicals are complex mixtures, often with poorly defined toxicological profiles. The profiles can become increasingly important because moving from a chemical with well-established risks to a chemical we know less about can make it difficult to define the hazard, so a higher risk will be assigned.
Extracts and Mixtures
Risk assessment of extracts or mixtures remains a complex problem. It is now recognized that significant data gaps exist in the area of mixtures toxicology, and these can complicate accurate risk assessments.6
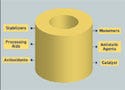
|
Figure 2. (click to enlarge) Potential extractables from polymeric biomaterials could migrate into the surrounding environment. |
It is difficult to judge the leaching risks associated with one pure substance; it is even more difficult if a solution or extract is a complex mixture of different compounds. Most analytical chemists are acutely aware that leachable residue is likely to be a blend of different chemicals (see Figure 2). The resulting biological effect of combined exposure to several agents can be characterized as additive, supraadditive (synergistic), or infraadditive (antagonistic). Another type of interaction, potentiation (a special form of synergism), may be observed. In cases of potentiation, one of two agents exerts no effect upon exposure. But when exposure to both agents together occurs, the effect of the active agent is increased.
The assumption is that compounds with similar metabolic pathways or even with similar structures will have an additive effective. Sometimes, a small change in chemical structure produces sharply different toxicological effects. In addition, there is the possibility that mixtures will have a synergistic effect (i.e., far greater than additive, so that the risk to humans is magnified). Or the effect could be antagonistic, for which the various residues cancel each other out.
When specific toxicological information is unavailable for a particular chemical, a modeling method customary to predictive toxicology can be used to ascertain potential hazards. OEMs use both qualitative and quantitative modeling methods relating chemical structure to biological activity. These methods, called structure-activity relationship (SAR) analyses, have been applied to the prediction and characterization of chemical toxicity.7
SARs are basic to toxicological investigations and are used in risk assessment. The fundamental premise is that the structure of a chemical implicitly determines its physical and chemical properties and reactivities. The properties, in interaction with a biological system, determine its biological and toxicological properties.6
SARs are accepted and provide great benefit; however, there are limitations. There is no single SAR test capable of predicting a particular activity or property for all compounds. A provision for the use of SAR to reduce testing needs is included under EPA's high production volume program.8 Under this program, a chemical category is defined as “a group of chemicals whose physicochemical and toxicological properties are likely to be similar or follow a regular pattern as a result of structural similarity.” The similarities should be based on a common functional group, common precursors, or breakdown products (resulting in structurally similar chemicals). The goal of developing a chemical category is to use interpolation and extrapolation to assess chemicals rather than to conduct additional testing with animals. The specific concern centers on animal welfare and the goal is to minimize the use of animals in the testing of chemicals.
The staff of the Office of Science and Engineering Laboratories (OSEL) at FDA has been responsible for conducting risk assessments on compounds released from medical device materials.9 OSEL staff were involved with the development of EN/ISO 10993-17, which established allowable limits using health-based risk assessment. The standard describes a process for determining an allowable or TI for a single chemical entity. However, it does not provide a good way to evaluate the toxicological significance or biological effect of a solution or extract containing a complex mixture of a number of different chemicals. Synergistic or antagonistic effects are not accurately determined or predicted.
The method outlined in EN/ISO 10993-17 (Method for the Establishment of Allowable Limits for Leachable Substances) was used by CDRH to establish TI for diethylhexyl phthalate (DEHP) released from PVC medical devices. “The safety assessment approach used by FDA/CDRH to derive the TI values is essentially identical to the method used by other regulatory agencies and advisory bodies to establish health protective exposure levels for DEHP (and other compounds).”10 The process is used to ascertain the safety of DEHP, set the precedence for this approach, and evaluate the safety or risk of exposure to extracted chemicals.
This process works well when dealing with a single chemical entity. However, as pointed out previously, antagonistic and synergistic effects are not accurately determined or predicted when multiple chemicals have been extracted. For this reason, biocompatibility tests listed in EN/ISO 10993–series standards should be used to complement a risk assessment process as described in EN/ISO 10993-17.
A second international standard, ISO 14971, “Medical Devices—Application of Risk Management to Medical Devices,” gives guidance with respect to evaluation of toxicological hazards.11 Annex I, “Guidance on Risk Analysis Procedure for Biological Hazards,” also provides suggestions for toxicological hazards caused by chemical constituents with the potential for biological harm. According to the standard, three major factors can be used to estimate toxicological risks, as follows:
The chemical nature of the materials.
Prior use of the materials.
Biological safety data.
The amount of data required and the extent of the investigation depend on the intended use or purpose and on the nature and duration of patient contact. Therefore, material intended for manufacturing an implantable device requires a more extensive investigation than a surface device contacting intact skin.
Collectively, knowing the material's composition, including additives and processing aids, prior use of the materials in a predicate device or similar device, and biological safety tests should provide predictive evidence of any toxicological hazard to patients. Although EN/ISO 10993-17 can be used to establish allowable limits for individual chemicals, biological safety tests when used to complement the risk assessment can give another measure of assurance.
In practice it is not possible to carry out complete chemical characterization of a complex mixture obtained from extracts of device materials. Therefore, the integration of chemical and biological information is critical to any assessment of the toxicity of complex mixtures. EN/ISO 10993-17 deals with establishing allowable limits for each individual chemical. ISO 14971 relies on biological safety data as one of the factors to evaluate toxicological hazards. In combination, appropriate biological and chemical tests provide a way to deal with some of the weaknesses of assessments of complex chemical substances.
Biological safety data provide another level of predictive evidence that none of the extracted chemical substances are potentially harmful to patients. In vitro tests, such as cytotoxicity and hemolysis, provide predictive evidence that extracted substances singularly and collectively are not toxic to mammalian cells.
In vitro tests enable a large number of combinations of chemicals to be assayed using a single test article. They are very useful in studies of acute toxicity and also biotransformation products of extractables. The sample extract or mixture is treated as a whole and tested as-is. Supporting data derived from in vitro and in vivo biological tests can help risk assessors make meaningful predictions as to likely human response. Cell studies can help identify the mechanism by which a substance has produced an effect in the animal bioassay. These tests have the ability to predict any unexpected potentiation or synergistic effects not accounted for by EN/ISO 10993-17 that may result in toxicity.
Conclusion
A comprehensive chemical characterization program that integrates the evaluation of extractables, device material stability, and toxicological risk assessment provides predictive evidence of safety and effectiveness of the device and all its constituents. It is important to give consideration to any potential biological or chemical interactions between the biological environment and the device.
The integration of chemical and biological information is critical to the assessment of toxicity of complex mixtures or device extracts. The guidance provided by EN/ISO 10993-17 and ISO 14971 has made it clear that together, biological safety tests, knowledge of the material's composition (including additives and processing aids, and prior use of the materials) in a predicate device or similar device should provide predictive evidence of any potential toxicological hazard to patients.
|
The value of risk assessments has long been recognized by international organizations and now EN/ISO 10993. In the risk assessment process, a decision must be made related to risk versus benefit. Once risk assessment has been completed, the focus turns to risk management. Part 17 of EN/ISO 10993 states that “manufacturers and processors may use the allowable limits derived to optimize processes and aid in the choice of materials in order to protect patient health.” Decisions should be made using the results of risk assessment, biological safety testing, and safe clinical use of predicate devices as described in ISO 14971. When coupled or linked to biological safety testing, a successful biological safety assessment becomes a highly useful decision-making tool.
Dave Albert is a senior scientist at NAMSA's Ohio laboratory. Reach him at [email protected]. Amy Hoffmann serves as a technical specialist supporting NAMSA's Ohio chemistry department. She can be contacted at [email protected].
References
1. ISO 10993-17:2002, “Biological Evaluation of Medical Devices—Part 17: Establishment of Allowable Limits for Leachable Substances” (Geneva: International Organization for Standardization, 2002).
2. ISO 10993-1:2003, “Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing” (Geneva: International Organization for Standardization, 2003).
3. “Risk Assessment in the Federal Government—Managing the Process,” National Research Council (Washington, DC: National Academy Press, 1983).
4. Guidance Note from 5 EC Medical Devices Directive—Guidance on the Biological Safety Assessment (Bootle Merseyside UK: UK Competent Authority, January 2006): 1–10.
5. VL Reynolds, “Applications of Emerging Technologies in Toxicology and Safety Assessment,” International Journal of Toxicology 42 (2005): 135–137.
6. DE Albert, B Kanegsberg, and E Kanegsberg, “Toxicological Risk Assessment for Medical Devices—What Is It?” Controlled Environment 8, no. 10 (2005): 32–33.
7. AP Worth, “The Tiered Approach to Toxicity Assessment Based on the Integrated Use of Alternative (Non-Animal) Tests” in Predicting Chemical Toxicity and Fate, ed. MTD Cronin, and DJ Livingstone (Washington, DC: CRC Press, 2004): 391–412.
8. JD McKinney et al., “The Practice of Structure Activity Relationships (SAR) in Toxicology,” Toxicological Sciences 56 (2000): 8–17.
9. “Chemical Hazard Data Availability Study: What Do We Really Know about the Safety of High Production Volume Chemicals?” (Environmental Protection Agency, Office of Pollution Prevention and Toxics, 1998).
10. “Safety Assessment of Di (2-ethylhexyl)phthalate (DEHP) Released from PVC Medical Devices” (Rockville, MD: FDA, 2004): 1–60.
11. ISO 14971:2000(E), “Medical Devices—Application of Risk Management to Medical Devices, Annex C: Guidance on Risk Analysis Procedures for Toxicological Hazards” (Geneva: International Organization for Standardization, 2000).
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like