Some of the devices nominated by our readers are small enough to travel through a blood vessel. Some are so large they fill an entire room. Some cost thousands of dollars but will stay in the body for 10 years, and some cost pennies and are designed to be thrown away after one use. This industry is characterized by innovators looking for the best way to engineer a solution to a problem. And device designers are noted for their ability to borrow ideas from other industries.
June 1, 2009
MD&DI 30th Anniversary
|
Blood and Cell Separator 1979
|
The CS-3000 system was the first automated blood and cell separator. The device was able to draw whole blood, keep the desired component, and return the remaining blood components to the donor. This product eliminated the risk of contamination associated with manual methods and enabled donors to give blood more frequently. It also made it possible for patients to receive blood from fewer donors.
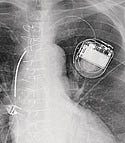
Implantable Cardioverter-Defibrillator 1980
|
Photo courtesy of MEDTRONIC INC. |
An implantable cardioverter-defibrillator (ICD) is a small battery-powered electrical impulse generator that is implanted in patients who are at risk of sudden cardiac death due to ventricular fibrillation. The device is programmed to detect cardiac arrhythmia and correct it by delivering a jolt of electricity. In current variants, the ability to revert ventricular fibrillation has been extended to include atrial and ventricular arrhythmias as well as the ability to perform biventricular pacing in patients with congestive heart failure or bradycardia. ICDs were pioneered at Sinai Hospital in Baltimore in 1969, but it was 11 years later that a patient first received treatment. Another decade of research went into the development of an implantable defibrillator that would automatically sense the onset of ventricular fibrillation and deliver an electric countershock within 15–20 seconds, converting the rhythm to sinus rhythm. Levi Watkins Jr., MD, implanted the first functioning device in February 1980 at Johns Hopkins Hospital.
Intra Articular Arthroscopic Shaver System 1980
|
|
Images courtesy of SMITH & NEPHEW |
Arthroscopic shavers are used in orthopedic procedures to remove tissue and reshape a patient's anatomy. A surgeon may use an arthroscopic shaver to remove bone or cartilage and other soft tissue from a patient's joint, or in procedures such as septoplasty (sinus reduction). The shavers include a rotating burr housed within a rigid insertion tube but exposed to body tissue through a small aperture in the side or end of the insertion tube. Suction is applied through the insertion tube so that debrided tissue can be sucked into the tube and removed from the body. This device contributed significantly to a radical transformation of orthopedic surgery. Prior to introduction, arthroscopy was mainly a diagnostic tool. This device was an essential development for minimally invasive orthopedic surgery.
Cochlear Implants 1980
|
Image courtesy of COCHLEAR LTD. |
In 1980, giving deaf people the ability to hear became a real possibility. William House, MD, performed the first cochlear implant on a child using a 3M/House device. Around the same time, Australian Paul Trainor began developing a cochlear implant, known as the Nucleus Multichannel Cochlear Implant. The device was first implanted in a human in 1982. FDA approved the 3M/House device for deaf adults in 1984 and the Nucleus implant in 1985. Today, one-year-old children are eligible for cochlear implants. The Nucleus Freedom (pictured above) is the modern version of Trainor's original design. It uses an electrode to offer a finer resolution of sound, as well as a sound processor that features four computers built on a microchip. At end of 2006, FDA reported that more than 112,000 people worldwide had received cochlear implants.
Angioplasty Balloon Catheter 1980
|
Photo courtesy of MEDTRONIC INC. |
In the 1970s, German cardiologist Andreas Gruentzig pioneered coronary angioplasty with the development of the double-lumen dilation catheter that used an inflatable balloon. The angioplasty balloon catheter, used for percutaneous coronary intervention, has both saved and improved the lives of patients. Before it hit the market in 1980, vessel bypass surgery was the most viable way to repair blocked vessels but carried more risks, pain, and cost. In addition to relieving chest pain and helping to prevent heart attacks, the device's significant potential has pulled dozens of medical device companies into the catheter space.
Personal Glucose Meter 1980
|
Image courtesy of HOME DIAGNOSTICS |
The first portable glucose meter was approved in 1969, but improvements to the technology cannot be emphasized enough. One of the most significant steps in the treatment of diabetes was moving the glucose testing from the hospital to the home. The first personal glucose meter was developed by Miles Laboratories Inc. (later purchased by Bayer). Since the early 1980s, these home-use devices have experienced steady improvements. For example, portable glucose meters with memory have become critical to diabetes care, because they enable diabetics to keep a record and observe trends and patterns. Event markers, digital user interfaces, and no-coding technology have also advanced the treatment standard. The modern meter, such as the True2go glucose meter shown here, can weigh less than an ounce.
Pulse Oximeter 1981
|
Photo courtesy of NELLCOR |
The first pulse oximeter was introduced in the United States in 1981. Rather than drawing blood, the device provided a noninvasive way to measure a patient's oxygen saturation level via wavelength measurements. In the 1980s, manufacturers of pulse oximeters introduced much smaller technology that was less expensive and easier to use. The Nellcor Pulse Oximeter (pictured) has evolved from its beginnings 25 years ago into a range of different products, from handheld devices that conduct spot check measurements to compact monitors that use nonadhesive specialty sensors for patients with fragile skin.
Laryngeal Mask Airway 1981
|
Image courtesy of AMBU INC. |
In 1981, anesthesiologist Archie Brain invented the Laryngeal Mask Airway (LMA), a device that establishes an unobstructed airway in unconscious patients or patients under anesthesia. Brain was looking for a device that could replace endotracheal intubation, a procedure that can cause trauma or unwanted reflux responses in patients. Since Brain's LMA was first used in a hospital in 1988, it has become a staple in operating rooms and ambulances. Many versions of the device exist today including the AuraOnce Disposable Laryngeal Mask (pictured above), which comes in a variety of sizes to fit a range of patients. LMAs have been used in millions of procedures.
EXCEL and PAB IV Containers 1983
|
Photo courtesy of B. BRAUN MEDICAL |
Since July 12, 2002, FDA has encouraged the reduction of patient exposure to Di(2-ethylhexyl)phthalate (DEHP) and recommends replacing DEHP-plasticized PVC medical products with products that contain alternative materials or plasticizers. This is particularly true for IV containers, for which plasticizer leaching may be a critical concern. Although the exact risk presented by DEHP is yet to be determined, many manufacturers took steps long before FDA made the recommendation to provide users with DEHP-free products. Manufacturer B. Braun Medical has offered a line of DEHP-free and PVC-free containers since 1983. Its signature products are the Excel and PAB IV containers. Plastic IV containers were first used in the 1950s.
Automated External Defibrillator 1985
|
Image courtesy of DEFIBTECH |
The automated external defibrillator (AED) has been instrumental in saving lives since it was first developed in 1985. Studies have shown that early defibrillation with an AED can dramatically increase survival rates—up to 70%. The device, which uses electricity to stop cardiac arrhythmia and help the heart reestablish a solid rhythm, has become so valuable that CPR training courses often include a segment on their use. These days, AEDs are ubiquitous: commercial aircrafts as well as police, fire, and EMT vehicles usually carry them. FDA approved the first home-use AED in 2004.
Digital Hearing Aid 1987
|
Image courtesy of INSOUND MEDICAL INC. |
A digital hearing aid isn't automatically superior to analog devices. But starting with the creation of the Nicolet Phoenix—the first digital hearing aid—in 1987, the devices have become increasingly sophisticated. Much of this owes to digital signal processing technology, which has allowed manufacturers to enhance features and provide users with more comfort and higher-quality hearing. For example, digital hearing aids can drastically reduce feedback while the listener is wearing the device and enhance speech based on temporal or spectral content. The Lyric is the only extended wear hearing device on the market—it can be worn continuously for up to 120 days.
Safety Needles and Syringes 1988
|
Photo courtesy of BECTON, DICKINSON AND CO. |
During the late 1980s and into the 1990s, the most frequent cause of bloodborne infections in healthcare settings was a needlestick. Safety needles have come a long way to help prevent such risks. The safety mechanism is often built into the needle. The needle can be manually attached to a conventional syringe or a commercially prepared prefilled syringe. Both active and passive methods are available. For the active system, the clinician activates the safety mechanism after the injection. In a passive system, the needle is automatically shielded after injection. Devices that often incorporate safety mechanisms include IV connectors, needle guards, sheathed syringes, needle-recapping products, blood-drawing devices, IV catheters, and needleless injection devices. In 1988, Becton Dickinson introduced the BD Safety-Lok, the first safety-engineered syringe. In 2000, the company launched the Safety Compliance Initiative, a nationwide education program to raise awareness about the risk of accidental needlesticks and to help healthcare institutions comply with federal mandates for safety-engineered devices.
Demineralized Bone Matrix Gel 1991
|
Photo courtesy of OSTEOTECH INC. |
In 1965, Marshall Urist identified the process of osteoinduction and ultimately discovered the first bone morphogenetic protein in demineralized bone. Orthopedic manufacturers later built on Urist's research to provide surgeons with off-the-shelf products for bone healing. In 1991, Oseotech Inc.'s Grafton demineralized bone matrix (DBM) Gel, made from allograft bone, was the first product of its kind to earn approval for use in musculosketal surgery. Today, Oseotech says that its Grafton DBM products have been used in more than 1 million surgical procedures.
Ventricular Assist Device 1992
|
Image courtesy of ABIOMED |
A ventricular assist device (VAD) is a mechanical pump that helps a weak heart pump blood through the body. It is often called a “bridge to transplant” because it can help patients survive until they get a new heart. The BVS 5000, a biventricular assist system manufactured by Abiomed, was the first VAD to earn FDA approval. It has supported thousands of patients since entering the market. Most recently, VADs have evolved to provide long-term support to patients with congestive heart failure.
Smart Infusion Systems 1992
|
Image courtesy of B. BRAUN MEDICAL |
Infusion pumps have been around for at least 30 years. But certain patient safety–related features, such as tools to ensure that patients receive the correct dosage, didn't appear until decades later. In 1992, Kendall McGaw developed the Horizon infusion system (now part of B. Braun Medical's pipeline)—the first system with a dose-rate calculator. Future iterations added more safety features, including a drug library inside the pumps (1994) and an integrated bar coding system (2001). Such advances, which are also found in the modern Outlook Safety Infusion System pictured here, have helped significantly reduce pump-related IV medication errors.
Headless Cannulated Bioabsorbable Interference Screw 1994
|
Photo courtesy of ALASKA ORTHOPEDIC LABS |
The headless cannulated interference screw has replaced many metal screws for anterior cruciate ligament (ACL) reconstruction surgery in the knee. The titanium screw was released in 1991 and was followed by a bioabsorbable screw three years later. The advantage of the bioabsorbable screw is that the body resorbs the polymer, replacing it with bone, so there is no need for a second surgery to remove the screw (as sometimes is the case when using metal screws). To accelerate reincorporation rates, the screw has been made with human cortical and bovine bone as well. Surgeons are also now using the screws for posterior cruciate, lateral collateral, and medial collateral ligament (MCL) procedures. Its use has led to the development of other cannulated interference screws and anchor devices for applications in the wrist, elbow, shoulder, hip, and ankle.
Palmaz-Schatz Balloon Expandable Stent 1994
|
Image courtesy of CORDIS CORP. |
The Palmaz-Schatz balloon expandable stent was a cutting-edge device that changed the game for coronary artery obstructions. The bare-metal stent was approved in August 1994 and made it clear that the new wave of treatment (stents) solved problems that balloon angioplasty alone could not. Johnson & Johnson held a critical patent relating to inflation-deployed stents, and this device enjoyed a period of market dominance once it debuted. The treatment options this device provided were an important stepping stone to the drug-eluting stent.
Medical Lasers 1995
|
Image courtesy of ABBOTT LABORATORIES |
Even now, many industry observers say that the surface has only been scratched with medical lasers and their potential. Medical lasers (light amplification by stimulated emission of radiation) use focused light sources to treat or remove tissue, and they are used for a variety of vision, dental, cosmetic, and general surgery procedures. One benefit for surgical procedures is less bleeding; heat from lasers cauterizes blood vessels, which leaves medical personnel with less blood to deal with compared with scalpels. Perhaps the most popular application is LASIK, a type of refractive laser eye surgery to correct myopia, hyperopia, and astigmatism. LASIK surgery has transformed outcomes for patients suffering from these conditions. FDA approved the first excimer laser in 1995.
Angio-Seal 1996
|
Photo courtesy of ST. JUDE MEDICAL |
The Angio-Seal is a breakthrough vascular closure device that uses bioabsorbable components to mechanically seal punctures in the femoral artery after a catheterization procedure. The quick seal occurs in three easy steps—locate, set, and seal—and is created by sandwiching the arteriotomy in the tissue tract between a copolymer anchor and a collagen sponge. In addition to providing patients with a shorter hospital stay, the seal dissolves within 60 to 90 days. The device was developed by Kensey Nash Corp., which licensed it to St. Jude Medical.
LightCycler Real-Time PCR 1998
|
Photo courtesy of ROCHE APPLIED SICENCES |
Molecular diagnostics have paved the way toward individualized medicine. The technology enables point-of-care diagnoses for infectious diseases, meaning infected patients can be identified quickly, enabling immediate treatment and protection for those at risk. One of the best-known devices is the LightCycler Real-Time polymerase chain reaction (PCR) System from Roche Diagnostics. Using PCR, scientists can take a specimen containing a minute amount of genetic material, repeatedly copy a selected region from it, and within hours, generate a sample sufficient to perform a variety of tests. PCR is versatile. Many types of samples (e.g., blood, skin cells, saliva, hair) can be analyzed for nucleic acids. Any sample used for PCR must contain the DNA strand encompassing the region to be amplified.
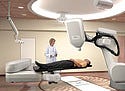
CyberKnife Robotic Radiosurgery System 1999
|
Photo courtesy of ACCURAY |
The CyberKnife is a miniature linear accelerator mounted to a robotic arm. It noninvasively delivers concentrated beams of radiation to a targeted tumor from multiple positions and angles. The tumor receives a cumulative dose of radiation high enough to control or kill the tumor cells while minimizing radiation exposure to surrounding healthy tissue. It delivers to almost all parts of the body, particularly for surgically complex tumors. In 1999, the device was approved for treatment of tumors in the head and base of skull. In 2001, FDA cleared enhancements to the CyberKnife System for tumors anywhere in the body.
da Vinci Surgical System 1999
|
Image courtesy of INTUITIVE SURGICAL |
The da Vinci surgical system has made it possible to treat a broader range of conditions with a minimally invasive approach. The system's microchip technology and 3-D optics enable surgeons to perform complex procedures by making tiny incisions. The da Vinci offers users greater precision, an increased range of motion, improved dexterity, and enhanced visibility. Because of the device, patients may experience less pain, less scarring, reduced risk of infection, and a faster recovery time. The surgical system has been used to treat heart conditions, prostate cancer, endometrial cancer, morbid obesity, and mitral valve regurgitation.
PillCam 2001
|
Image courtesy of GIVEN IMAGING |
Is your digestive tract ready for its close-up? The PillCam is a capsule that houses a miniature video camera, lights, a transmitter, and batteries. Once a patient swallows the pill and it passes through the digestive tract, it takes photos of the small intestine and sends them to a small recorder affixed to the patient's belt. This technology has helped patients avoid invasive and painful endoscopic diagnostic procedures just by swallowing a pill. It also allows the entire small bowel to be viewed (endoscopes allowed physicians to see only the upper part of the small bowel).
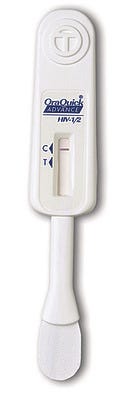
OraQuick Advance Rapid HIV-1/2 Antibody Test 2002
|
Image courtesy of ORASURE TECHNOLOGIES INC. |
Major progress has been made since the first AIDS-related diagnostic test was commercialized in 1988. In 2002, the OraQuick Advance Rapid HIV-1/2 Antibody Test became the first rapid HIV test to earn FDA approval. In clinical studies conducted by the manufacturer, OraSure Technologies Inc., the test correctly identified 99.6% of people who were infected with HIV-1 and 100% of people who were not infected with it. The test provides results for patients in about 20 minutes. This is key because with previous tests, which took several days to process, a significant number of patients never returned to the clinic to learn whether or not they were infected. The test can be stored at room temperature, requires no specialized equipment, and can be used in both laboratory and nontraditional clinical settings.
Drug-Eluting Stent 2003 (U.S. release)
|
Image courtesy of ABBOTT LABORATORIES |
Blocked coronary arteries used to be addressed by coronary artery bypass graft surgery. Then came balloon angioplasty. But angioplasty was often plagued by restenosis, a narrowing of a blood vessel that restricts blood flow. The stent was the next development, and although it improved outcomes, it also increased the risk of thrombosis (clotting) and occlusion of the stent. Eventually, stent developers wondered whether the device itself could be a conduit for medication. Enter the drug-eluting stent, which was first approved in Europe in 2002. It has reduced restenosis rates and provided significantly better clinical outcomes for patients. The drug-eluting stent has also had bigger implications for the medical device industry—it has helped usher in the era of combination products. The marriage of devices and drugs is still changing the industry as regulators and developers continue evaluating how these products are dealt with.
Sidne Voice Activation System 2003
|
Photo courtesy of STRYKER |
As the first FDA-approved Bluetooth-ready medical device, Sidne represents where the future of Bluetooth is headed in the device industry. The system, which stands for Stryker integrated device network, uses voice recognition to give surgeons control over endoscopy equipment in the operating room without touching a button. Bluetooth technology eliminates tangled cable wires from the control unit to devices on the operating table, and a wireless headshot gives surgeons the freedom to move, as well as answer and dial calls through the hospital. The device is also modular, allowing hospitals to customize the control package based on their needs.
LifePort Organ Transporter 2003
|
Photo courtesy of WI MEDICAL DEVICE DEVELOPMENT |
The method of storing and transporting organs has traditionally involved placing an organ in a cooler filled with ice—until the LifePort Organ Transporter came into the picture. The product uses mobile machine perfusion to improve transplant outcomes, allowing the use of more organs and lowering the number of organs discarded. The process involves pumping a cold solution through the organ to reduce tissue damage even while the organ is in transport. The LifePort started as a transporter for kidneys and has been so successful that the product is branching out into use for the heart, pancreas, liver, lung, and intestines.
OxyMask 2005
|
Photo courtesy of SOUTHMEDIC INC. |
The OxyMask is designed to overcome the common side effects of closed oxygen masks such as mucosal drying, nose bleeding, facial sores, and the claustrophobic nature of the device. Its open oxygen system design eliminates CO2 rebreathing, allows patients to communicate more easily with clinicians, and enables drinking through a straw. A nasogastric tube can also be threaded through the mask, and a swivel elbow expands the device's flexibility by allowing left-to-right positioning of oxygen tubing. The mask also has a PVC-free version.
Pinnacle TPN Management System 2007
|
Photo courtesy of B. BRAUN MEDICAL |
In an improvement on patient safety, the Pinnacle system provides a safe and easy way to check, compound, and deliver total parenteral nutrition (TPN) to patients. The device controls intravenous formulations using amino acids, dextrose, electrolytes, and lipids for patients who rely on tube feeding or can't tolerate oral intake of food. The Pinnacle system streamlines the TPN process by combining automated compounding technology with a safety verification system and special software. The system can accurately measure and combine up to nine nutritional compounds and prepares one liter of TPN solution in less than one minute. Its safety-check software and bar coding ensure that solution is going to the correct patient.
Impella 2.5 Circulatory Support System 2008
|
Photo courtesy of ABIOMED |
The Impella 2.5 is a minimally invasive, percutaneous cardiac assist device that allows the heart muscle to rest and recover. Impella is designed to actively unload the left ventricle, reduce heart muscle workload and oxygen consumption, and increase cardiac output and coronary and end-organ perfusion. Impella received FDA 510(k) clearance in June 2008 and has been used to treat heart attack patients, patients undergoing high-risk angioplasty, peripartum cardiomyopathy, and viral myocarditis. It is 1∕100 the size of the heart. The device is approved in more than 40 countries, has been used to treat more than 1700 patients worldwide, and has been the subject of more than 50 peer-reviewed publications. The device was the recipient of a 2007 Medical Design Excellence Award.
Many key technologies got their start before MD&DI's inception and before current regulations. Here are a few that have made a significant contribution to the way medicine is practiced.
Vena Cava Filter 1973
|
Photo courtesy of BOSTON SCIENTIFIC CORP. |
Unique in healthcare disciplines is the fact that devices often draw inspiration from the outside world to solve medical problems. In oil pipelines, a cone-shaped filter traps sludge and debris. The geometry of the cone allows oil to continue flowing around its edges while concentrating the sludge in the center, whereas a flat screen, with sludge spread across it, could completely clog the pipeline. The vena cava filter is the same basic shape as that used in oil refining and is used to prevent life-threatening pulmonary embolisms.
Computed Tomography Scanner 1972
|
Photo courtesy of SIEMENS |
Since its introduction in the 1970s, computed tomography (CT) has become an important tool in medical imaging. It is the gold standard in the diagnosis of a number of different disease entities. CT produces data that are manipulated through a process known as windowing. It demonstrates bodily structures based on their ability to block the x-ray beam. Although historically the images were in the axial or transverse plane, orthogonal to the long axis of the body, modern scanners allow this volume of data to be reformatted in various planes or even as 3-D representations. The first commercially viable CT scanner was invented by Sir Godfrey Hounsfield in Hayes, UK, at EMI Central Research Laboratories. Hounsfield conceived his idea in 1967, and it was publicly announced in 1972. Allan McLeod Cormack of Tufts University in Massachusetts independently invented a similar process, and both Hounsfield and Cormack shared the 1979 Nobel Prize in medicine. The original prototype took 160 parallel readings through 180 angles, each 1˚ apart, with each scan taking about five minutes. The images from these scans took 2.5 hours to be processed by algebraic reconstruction techniques on a large computer.
Magnetic Resonance Imaging 1977
The first magnetic resonance (MR) image was published in 1973 and the first study performed on a human took place on July 3, 1977. MR imaging was developed from the study of nuclear magnetic resonance. In its early years, the technique was referred to as nuclear magnetic resonance imaging. The technology is used to visualize the internal structure and function of the body. MR provides contrast between the different soft tissues of the body, making it especially useful in neurological, musculoskeletal, cardiovascular, and oncological imaging. Applications for MR technology continue to evolve. For example, recent applications in functional MRI measure changes in the brain, which could lead to more information about the nature of diseases, such as stroke. They are also being moved into the OR to take images during surgical procedures.
Hemodialyzers and Dialysis Machines 1800s–1970s
These devices provide an artificial replacement for lost kidney function due to renal failure. In hemodialysis, the patient's blood is pumped through the blood compartment of a dialyzer, exposing it to a semipermeable membrane. The cleansed blood is returned via the circuit back to the body. Ultrafiltration occurs causing water and dissolved solutes to move from blood to dialysate and allows the removal of excess fluid.
Artificial Pacemaker 1960s–1970s
|
Photo courtesy of ST. JUDE MEDICAL |
The development of the pacemaker arguably gave legitimacy to the discipline of medical device engineering. Its history is linked to famous developers, such as Earl Bakken, Manuel Villafaña, and Wilson Greatbatch. Modern pacemakers have multiple functions. The most basic monitor the heart's native electrical rhythm. When the pacemaker fails to sense a heartbeat within a normal beat-to-beat time, it stimulates the ventricle with a short low-voltage pulse. This activity continues on a beat-by-beat basis.
Copyright ©2009 Medical Device & Diagnostic Industry
You May Also Like