Medical Device & Diagnostic Industry Magazine MDDI Article Index
December 1, 2005
Medical Device & Diagnostic Industry Magazine Originally Published MDDI December 2005
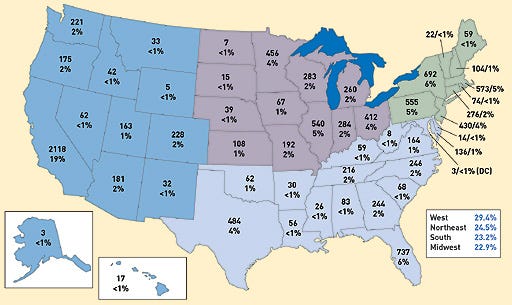
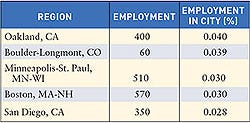
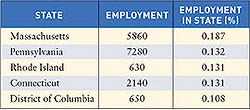
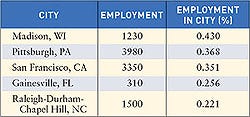
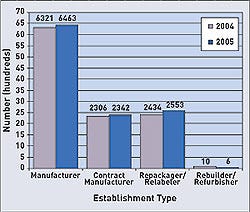
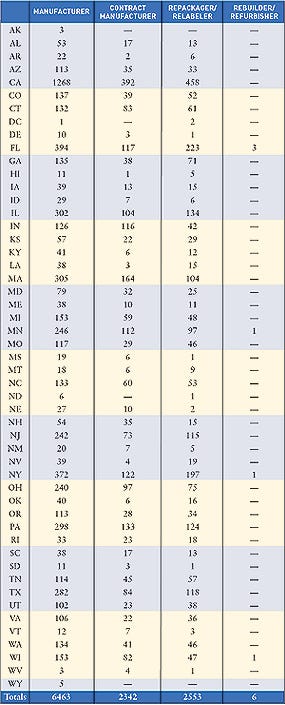
Nearly every state saw an increase in the number of registered device manufacturing facilities in 2005. Overall, the number of FDA-registered medical device establishments increased from 2004 (11,071) to 2005 (11,364). To submit registration and listing submissions, contact FDA at the following numbers: 240/276-0111; fax: 301/527-9187, or e-mail [email protected]. For policy questions, contact CDRH at 240/276-0110; fax 240/276-0140; or e-mail [email protected]. Click images to enlarge:
Copyright ©2005 Medical Device & Diagnostic Industry |
You May Also Like