April 1, 2007
MDEA 2007
Critical-Care and Emergency Medicine Products
Cool-Cap neonatal head-cooling system, manufactured by Olympic Medical Corp. (Seattle) and submitted by Natus Medical Inc. (San Carlos, CA). See description here.
|
Impella 2.5 circulatory support system, manufactured and submitted by Abiomed Inc. (Danvers, MA). The Impella 2.5 is the world's smallest ventricular assist device. It provides increased circulatory support during catheterization laboratory procedures such as high-risk angioplasty.
|
Organ Care system, manufactured by Transmedics Inc. (Andover, MA) and submitted by Farm Design Inc. (Hollis, NH). The Organ Care system is designed to maintain transplantable organs in a warm functioning state outside the body in order to optimize their health and permit continuous clinical evaluation.
Supply and design credit: Atlantic Industrial Models (Essex, MA), Design Formations (Blackstone Smithfield Industrial Park, RI), Dynamic Plastics (Chesterfield, MI), Farm Design, Forecast (Carlsbad, CA), and Vermont Composites (Brattleboro, VT).
Dental Instruments, Equipment, and Supplies
|
EtchMaster dental air abrasive etching instrument, manufactured and submitted by Groman Inc. (Margate, FL). EtchMaster is a disposable micro air abrasion tip for localized intraoral etching of tooth surfaces and prosthetics. Each EtchMaster tip is prefilled with abrasive powder and is powered and controlled by air via a simple chairside adapter.
Supply and design credit: Bailey Packaging (Cranford, NJ) and Samco Scientific Corp. (San Fernando, CA).
|
Ezlase diode dental laser system, manufactured and submitted by Biolase Technology Inc. (Irvine, CA). Ezlase is a dental laser system that uses semiconductor diode technology, a unique wavelength, and sterile, single-use fiber tips to perform soft-tissue clinical procedures with minimal bleeding and maximum patient comfort.
Supply and design credit: Liquidmetal Inc. (Lake Forest, CA), Morphix Design Inc. (San Clemente, CA), and Vista Dental Inc. (Racine, WI).
|
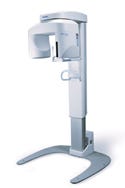
Orthoralix 8500 panoramic dental x-ray unit, manufactured by Ka Vo Dental Corp., Gendex Dental Systems (Des Plaines, IL) and submitted by Cesaroni Design Associates Inc. (Glenview, IL). The Orthoralix 8500 is a panoramic x-ray unit used to take dental x-rays. The panoramic system provides superior mechanical performance while producing excellent diagnostic image quality.
Supply and design credit: Cesaroni Design Associates and Profile Plastics Corp. (Lake Bluff, IL).
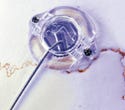
Thermoplastic Drill Template Set, manufactured by Institut Straumann AG (Basel, Switzerland) and submitted by Applied Dental Inc. (Mountain View, CA). See description here.
Supply and design credit: Applied Dental.
Finished Packaging
Ospol dental implant system packaging, manufactured by Ospol AB (Malmö, Sweden) and submitted by Avalon Technology AB (Solna, Sweden). See description here.
Supply and design credit: Avalon Technology AB.
General Hospital Devices and Therapeutic Products
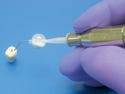
Episure AutoDetect epidural syringe, manufactured and submitted by Indigo Orb Inc. (Irvine, CA). See description here.
Supply and design credit: Apex Engineering (Torrance, CA) and Hi Tech Group Inc. (Anaheim, CA).
Mobile Clinical Assistant, manufactured by Intel Corp. (Beaverton, OR) and submitted by Whipsaw Inc. (San Jose). See description here.
Supply and design credit: Whipsaw Inc.
|
Odor-Absorbing Hydrocolloid wound dressing, manufactured and submitted by Avery Dennison Medical (Turnhout, Belgium). The Odor-Absorbing Hydrocolloid dressing captures wound malodors and absorbs exudate from lightly to moderately draining wounds. It can provide a protective, occlusive barrier that facilitates autolytic debridement, granulation, and epitheliazation.
Pinnacle total parenteral nutrition management system, manufactured and submitted by B. Braun Medical Inc. (Bethlehem, PA). See description here.
Supply and design credit: Arizona Medical Devices (Scottsdale, AZ).
SureSkin central catheter and IV dressing, manufactured and submitted by Euromed Inc. (Northvale, NJ). See description here.
Symbiq infusion system, manufactured and submitted by Hospira Inc. (Lake Forest, IL). See description here.
Supply and design credit: Design Concepts Inc. (Madison, WI) and Minnetronix Inc. (St. Paul, MN).
Vest airway clearance system, Model 205, manufactured and submitted by Hill-Rom Company, Inc. (Charleston, SC). See description here.
Implant and Tissue-Replacement Products
|
Gynecare TVT Secur system for treating stress urinary incontinence, manufactured and submitted by Ethicon Inc. (Somerville, NJ). The Gynecare TVT Secur system is a pubo-urethral sling for treating female stress urinary incontinence resulting from urethral hypermobility or intrinsic sphincter deficiency.
|
OrthoGlide medial knee implant, manufactured and submitted by Advanced Bio Surfaces (Minnetonka, MN). OrthoGlide is a cobalt-chrome medial knee compartment implant that reduces pain in osteoarthritic patients by providing a low-friction articulating surface. It can be implanted through minimally invasive outpatient surgery, with no bone cutting.
Supply and design credit: Doncasters Medical Technologies (Oregon City, OR).
|
StimLoc deep-brain lead immobilizer, manufactured and submitted by Image Guided Neurologics (Melbourne, FL). StimLoc is a permanent implant used to immobilize electrical leads used for deep brain stimulation for the treatment of Parkinson's disease, essential tremor, and dystonia.
Supply and design credit: Medtronic Neurological (Minneapolis), Reflex Medical Molding (White Bear Lake, MN), and Vertex Technology LLC (St. Paul, MN).
In Vitro Diagnostics
Piccolo Xpress chemistry analyzer, manufactured and submitted by Abaxis Inc. (Union City, CA). See description here.
Supply and design credit: James Toleman Industrial Design (Puget Ville, France).
|
Symphony one-touch histology slide preparation system, manufactured and submitted by Ventana Medical Systems Inc. (Tucson, AZ). The Symphony system automates the preparation of hematoxylin- and eosin-stained histology slides, resulting in high-definition slides that improve diagnostic visualization and provide exceptional discrimination of microanatomic detail.
Supply and design credit: Acu-Cast Technologies LLC (Lawrenceburg, TN), Bürkert Contromatic Corp. (Irvine, CA), CPC Colder Products (St. Paul, MN), Kerk Motion Products (Hollis, NH), Piper Plastics Inc. (Chandler, AZ), and Thermal Circuits Inc. (Salem, MA).
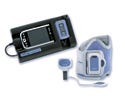
UltraCrit hematocrit measurement device, manufactured by Separation Technology Inc. (Altamonte Springs, FL) and submitted by Key Technologies Inc. (Baltimore). See description here.
Supply and design credit: CD Product Designs (Columbia, MD), Conductive Technologies Inc. (York, PA), and Key Technologies Inc.
Over-the-Counter and Self-Care Products
|
Conveen Optima silicone urisheath, manufactured and submitted by Coloplast A/S, Urology and Continence Care Div. (Kokkedal, Denmark). The Conveen Optima is a silicone urisheath for males suffering from urinary incontinence. Designed for easy application by gloved caregivers or by users with reduced hand dexterity, the device is appealing for both professional and personal-care markets.
|
Easypod subcutaneous injection device, manufactured and submitted by Merck Serono International SA (Geneva). Easypod is an electromechanical device for subcutaneous injection of medicinal products, and it is marketed for adult and pediatric self-administration of growth hormone.
Supply and design credit: Flextronics GmbH (Althofen, Austria) and PDD Group Ltd. (London).
Vicks Forehead thermometer, manufactured and submitted by Kaz Inc. (New York City). See description here.
Supply and design credit: Scott Henderson Inc. (Brooklyn, NY).
Radiological and Electromechanical Devices
Halo360 esophageal tissue ablation system, manufactured by Bârrx Medical Inc. (Sunnyvale, CA) and submitted by Stellartech Research Corp. (Sunnyvale, CA). See description here.
Supply and design credit: Accurate Injection Molds (Clinton Township, MI), Advanced Polymers Inc. (Salem, NH), All Flex Inc. (Northfield, MN), Stellartech Research, and TSI Inc. (Shoreview, MN).
|
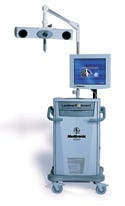
LandmarX Element endoscopic image guidance system, manufactured by Medtronic Navigation (Louisville, CO) and submitted by Medtronic ENT (Jacksonville, FL). The LandmarX Element endoscopic image guidance system is designed for use in functional endoscopic sinus surgery. The system provides an automated software flow and navigation instruments with rotating arrays.
Supply and design credit: Medtronic ENT.
RT Vue retinal imaging system, manufactured by Optovue Inc. (Fremont, CA) and submitted by Whipsaw Inc. (San Jose). See description on here.
Supply and design credit: Whipsaw Inc.
Rehabilitation and Assistive-Technology Products
|
Ness L300 leg neuroprosthesis, manufactured by Ness Ltd. (Ra'anana, Israel) and submitted by Bioness Inc. (Santa Clarita, CA). The Ness L300 is a noninvasive neuroprosthesis that delivers electrical stimulation to nerves and muscles in the leg, improving gait in those suffering from foot drop associated with central nervous system injury or disease.
Supply and design credit: Aran Ltd. (Caesarea Business Park, Israel) and Jonathan Bar-Or Industrial Design Ltd. (Pardes Hanna, Israel).
|
Pilot Walker enhanced mobility aid, manufactured by Full Life Products LLC (Moorestown, NJ) and submitted by Definitive Design (Langhorne, PA). The Pilot Walker is an enhanced mobility aid designed and engineered to meet the needs of patients with special mobility challenges.
Supply and design credit: Definitive Design.
|
Proprio Foot motor-powered prosthesis, manufactured and submitted by Ossur (Reykjavik, Iceland). The Proprio foot is the first artificially intelligent, motor-powered prosthetic foot. Suited for below-knee amputees, it offers physiological benefits such as automatic ankle flexion that are as close as current prosthetics can get to the human foot.
Supply and design credit: Dynastream Innovations Inc. (Cochrane, AB, Canada).
Xtensor upper extremity therapy device, manufactured and submitted by Clinically Fit Inc. (Melville, NY). See description here.
Supply and design credit: HS Design Inc. (Gladstone, NJ).
Surgical Equipment, Instruments, and Supplies
Mammotome MR breast biopsy system, manufactured and submitted by Ethicon Endo-Surgery (Blue Ash, OH). See description here.
Supply and design credit: Nupro Design Ltd. (Loveland, OH) and Strategix Vision (Bozeman, MT).
|
NexFrame deep-brain trajectory guidance system, manufactured and submitted by Image Guided Neurologics (Melbourne, FL). The NexFrame is a skull-mounted trajectory guide used to implant electrical leads for deep brain stimulation. It is a single-use device delivered to the customer prepackaged and EtO sterilized.
Supply and design credit: Fredrick Haer & Co. (Bowdoin, ME), Reflex Medical Molding (White Bear Lake, MN), Surgical Navigation Technologies (Minneapolis), and Vertex Technology LLC (St. Paul, MN).
|
Pascal photocoagulator, manufactured and submitted by OptiMedica (Santa Clara, CA). The Pascal photocoagulator is an integrated semiautomatic pattern-scan laser photocoagulation system designed to treat retinal diseases using a single shot or a predetermined pattern array of up to 56 spots within 0.6 seconds.
Supply and design credit: National Instruments (Austin, TX), Out of the Blue Design (San Francisco), and RSID Studio (San Francisco).
|
Stryker Precision Saw, manufactured and submitted by Stryker Instruments (Kalamazoo, MI). The Stryker Precision Saw is an oscillating-tip surgical saw for cutting bone, typically in total knee arthroplasty. The design permits the surgeon to hold the stationary component of the blade, resulting in unprecedented control and precision.
Supply and design credit: Bihler Weissenbach GmbH and Co. KG (Weissenbach, Austria), Ruetschi Technology (Muntelier, Switzerland), Sandvik Materials Technology (Sandviken, Sweden), Smithstown Light Engineering (Shannon, Co. Clare, Ireland), and XL Engineering (Niles, IL).
Does Your Company Have a Winning Design?
The Medical Design Excellence Awards program is open worldwide to companies and individuals involved in the design, engineering, manufacture, or distribution of finished medical devices or medical packaging. For the 2008 awards, all entered products must be commercially available by December 31, 2007.
Important dates:
November 12, 2007: Early Bird Deadline for 2008 Entries
December 17, 2007: Standard Deadline for 2008 Entries
For more information, please visit www.mdeawards.com.
You May Also Like