May 8, 2017
Medtronic initiated a new recall and expanded an earlier recall, both related to the HeartWare Ventricular Assist Device the company acquired last year.
Amanda Pedersen
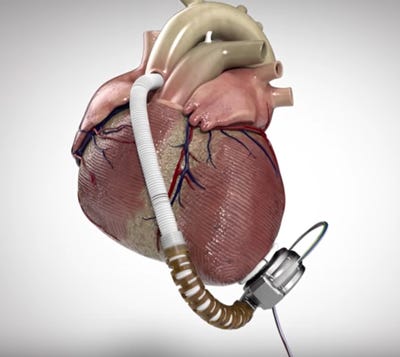
Medtronic acquired the HVAD through its $1.1 billion purchase of HeartWare last year.
Medtronic's HeartWare Ventricular Assist Device (HVAD) could have more problems than previously thought. The company expanded an earlier recall of HVAD controllers, and initiated a new recall of a driveline splice kit used to repair the HVAD's driveline once an electrical break has been identified.
The HVAD is designed to help deliver blood from the heart to the rest of the body. The device, which Medtronic acquired through its $1.1 billion purchase of HeartWare last year, is used in patients who are at risk of death from end-stage left ventricular heart failure and who are waiting for a heart transplant. The system includes a pump, which is implanted in the space around the heart, and a driveline cable that goes through the skin and connects the pump to an external controller that regulates the speed and function of the pump.
Only nine driveline splice kits are included in the new recall, and they were issued between April 2010 and March 2015 to repair previous HVAD driveline cable connector assembly issues.
These kits are being recalled because of a design problem that would prevent the repaired cable assembly from withstanding excessive force or pull, such as accidental dropping of the controllers or snagging of the driveline cables. The excessive force or pull could cause damage to the cable assembly and interrupt the electrical connection, according to FDA.
An interruption in electrical connection may cause the heart pump to stop, which may lead to serious adverse health consequences, including death, the agency said.
In March, Medtronic told affected customers via an urgent recall notice that there is a new HVAD driveline splice kit available.
The company also decided to expand its earlier recall of HeartWare controllers to include an additional product code. The controllers were recalled due to a loose power connector, which may cause the rear portion of the pump's driveline connector to become separated from the front portion of the driveline connector. A loose connector may allow moisture to enter the controller, causing corrosion, electrical problems, reduced speaker volume, and connection failures. If the speaker volume is decreased, FDA noted, the patient may not hear the alarm.
All HeartWare controllers are part of the recall, and 8,343 devices are included.
Amanda Pedersen is Qmed's news editor.
About the Author(s)
You May Also Like


