Gas plasma is commonly referred to as the fourth state of matter. However, Pulitzer Prize–winning author Curt Surplee more accurately describes plasma as the first state of matter, because gas plasma is composed of the electrons and ionized species that are the building blocks of physical chemistry. Appropriate use of these energetic molecules enables custom reengineering of surface chemistry at low cost and high efficiency.
Not all types of plasma are equivalent. The difference between some naturally occurring plasmas can be quite distinct. For example, a lightning bolt contains an apparently high density of electrons, whereas a coronal mass ejection is better categorized by high temperature. Yet both are denoted as plasma.
The same is true for the plasma employed in manufacturing tasks (e.g., corona, flame, atmospheric, and low pressure). When choosing a plasma operation that’s right for the application, consider stability, form factor, and the necessary surface functionality.
Atmospheric and low-pressure plasmas produce the greatest density of energetic species. Atmospheric plasma jets use clean, compressed air for the cleaning and activation of metals and polymers. In most cases, treatment is line of sight, the residence time is short, and the jet nozzle is integrated in-line with relative ease.
Another clean, work-horse material is low-pressure gas plasma, also known as cold gas plasma. This method requires a controlled volume, vacuum pump, and a choice of gas or vapor species. The control over the gas composition enables the greatest versatility in surface chemistry addition, and the low-pressure method is suitable for batch operation and the treatment of complex 3-D geometry. Both the atmospheric jet and low-pressure plasma chamber provide angstrom-level precision in their surface treatments.
Energy Source
All plasma starts with an energy source, most commonly a pair of electrodes supplying radio frequency or microwave energy to a volume of gas. Molecules trapped within the high frequency (13.56 MHz) electric field attempt to flip-flop within the oscillating current. Some of these particles soon become excited, or ionized, as their electrons are shed and plasma is sustained. Some of these charged species become unstable or meta-stable molecules. To return to thermal equilibrium, energetic species form covalent bonds with other particles or surfaces present within a plasma chamber.
Rough vacuum systems are used to increase the intensity of energetic species while avoiding elevated temperatures. This makes low-pressure plasma best suited for modifying thermally sensitive materials such as plastics. At lower pressure, particles have larger distances to travel before their energy is lost in collision or friction. The operating pressure for most commercial low-pressure plasma processes is 50–500 mTorr.
An attractive attribute of low-pressure plasma in life sciences is the ability to tailor surface chemistry. Chemists and engineers introduce specific gas or vapor combinations into their plasma reactor. Liquid and gas flow is regulated by a respective mass flow controller. Only a modest number of species is typically required for complete exposure of a surface. The process is extremely lean and the consumables negligible in contrast to a wet or dip processes. The exact chemical formulation of gas plasma may vary from simple to complex, and there is rarely a single route to achieve a desired surface functionality.
Surface Modification
There are three primary variables that govern surface modification: plasma chemistry, energy per mol, and residence time. A judicious selection of these process conditions yields high quality surface treatment of both organic and inorganic substrates. A low-pressure plasma reactor is a complete chemistry toolbox for manufacturing.
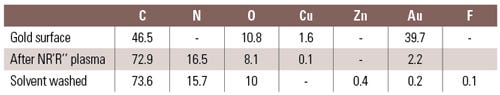
The study of plasma-modified materials is possible through a multitude of analytical and spectroscopic techniques. X-ray photoelectron spectroscopy (XPS), also known as electron spectroscopy for chemical analysis (ESCA), is able to quantitatively determine the chemical state of a surface to the topmost 10–100 angstroms. Table I illustrates the elemental fractions detected on a gold surface after the plasma deposition of an organic amine compound. The native gold surface is masked by a dramatic rise in nitrogen and carbon content. A suppressed gold signal suggests a modification much thicker than a few monolayers. This process of depositing ultrathin coatings using a plasma reactor is termed plasma enhanced chemical vapor deposition (PECVD).
Ultrathin coatings offer a permanence that other plasma methods may not provide because polymeric surfaces usually have some degree of chain mobility or rotation that effectively reduces the surface treatment over time. Reorientation of polymer chains disturb the accessibility of surface functionality.
Some materials such as elastomers and thermoplastics contain small molecules and low-molecular weight species such as plasticizer and oligomers. Trace amounts are able to migrate to the surface and effectively block the established surface groups. A substrate bound to a contiguous ultrathin layer is more likely to retain uninterrupted surface chemistry.
|
Table I. An x-ray photoelectron spectroscopy survey of elements on a gold surface shows before and after a gas-plasma amine chemistry. |
Table I also compares a plasma-coated gold surface before and after solvent wash. The elemental nitrogen content remains almost unchanged—confirming the surface layer is permanently bound to the substrate as opposed to a freestanding film. The chemistry of a surface is accessible to reaction with environmental or biological systems. The accessibility enables immobilization of protein and other communicative molecules. Some of the common compounds that can be grafted to a surface using plasma include amine, carboxyl, hydroxyl, vinyl, and thiol. Adsorption and conjugations of species can occur.
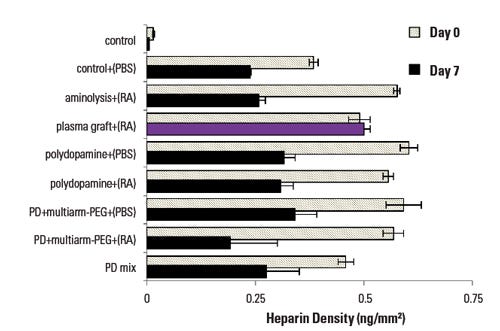
Figure 1 compares methods for loading heparin on an elastomeric copolymer. Only the plasma-grafted surface exhibited no loss in heparin density following seven-day incubation. All trials show initially good heparin loading compared with the control surface. Methods in which plasma was not employed experienced at least a 50% reduction in heparin over the period. The plasma-modified surface demonstrates process stability.
|
Figure 1. Heparin density is compared, following gas plasma conjugation and liquid-phase conjugation of a cardiovascular graft, after undergoing 7-day incubation in a bioreactor. |
Wet Versus Gas
Although wet chemistry methods exist for the modification and grafting of species, gas plasma offers superiority in cost, modification of inert surfaces, and scalability into commercial production. A gas- or vapor-phase plasma requires only marginal amounts of species for interaction with the surface. These have only a short residence in the reactor vessel before reaching the chamber exhaust.
A wet bath such as liquid-phase silanization, on the other hand, requires that a silane be dissolved in a solvent and then a clean substrate be added to the solution for durations sometimes exceeding hours.1 A liquid-phase silanization is not easy to control either, mainly due to difficulty in restricting the amount of water present in the solution. An absence of water results in incomplete monolayers and an excess of water results in homopolymerization or surface aggregation.2
In a gas-phase deposition using low-pressure plasma, moisture is controlled, eliminating unwanted surface formation. The high permeability of gas molecules allows function of nano-scale features or porous structures where access to the liquid solution is limited by capillary forces.
Surface Morphology
Varying the energy applied to gas plasma affects the surface morphology. At high energy, ablation dominates, and the substrate becomes rigorously etched or nano-roughened. At lower power regimes, gas plasma is a primarily additive process and the conditions are amenable to film formation.
|
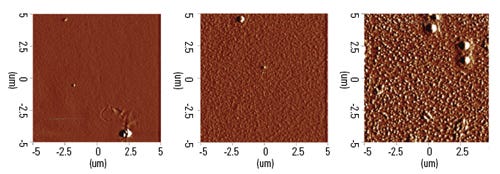
Figure 2. A 5 × 5–μm contact mode AFM image of topography demonstrates the different surface morphology resulting from continuous [left], low-pulsed [middle], and high-pulsed [right] plasma power conditions on a PECVD coating. |
An ultrathin PECVD coats surface features with an atomically smooth layer. If the energy is delivered in pulsed periods, the plasma yields nano-patterned film morphology. Figure 2 compares atomic force microscopy images of three PECVD fluorocarbon coatings on a glass microscope slide. The pulsed power conditions yield bumpy textures. In a PECVD of fluorocarbon, a surface pattern increases the surface area, thereby providing greater hydrophobicity.
Contact angle measurements using deionized water results in approximately 110° for the continuous power process and greater than 130° in the case of a pulsed power condition. Fluorocarbon coatings are noted for their properties of super hydrophobicity, resistance to protein adsorption, and anticorrosion.3 Morphology plays a big role in these surface interactions.
Another attribute of low-pressure gas plasma is its ability to develop custom PECVD surface chemistry that resembles conventional material systems. Polyethylene glycol (PEO) is a polyether compound with applications in manufacturing and medicine. Characteristics include biocompatibility, nonfouling, and nonimmunogenicity.4
Inspection via FTIR-ATR confirms an ether content of greater than 80%. The coating is both hydrophilic and resistant to protein adsorption. PECVD of a PEO-like film offers the advantages of being conformal, covalently bound, and chemically cross-linked for improved wear resistance. Other experiments have reported application of comparable PECVD chemistry in combination with lithographic patterning for directed cell growth.5 Cell attachment may be both assisted and inhibited by surface treatment.
Conclusion
Gas plasma for manufacturing isn’t new. Early adopters of large-scale commercial operations date back more than two decades. But gas plasma has remained one of industry’s best-kept secrets.
Today’s economic climate drives the need for a manufacturing process that is leaner, greener, and more efficient than ever before. Consumers demand lower cost and greater ecological responsibility. Medical and diagnostic devices are at the point of care. Product evolution drives greater accessibility to end-users and further into the realm of fast moving consumer goods. Surface solutions, such as gas plasma, can transform commodity plastics into smart materials. The operation is lean, efficient, and highly versatile.
References
CM Halliwell and AEG Cass “A Factorial Analysis of Silanization Conditions for the Immobilization of Oligonucleotides on Glass Surfaces,” Analytical Chemistry 73, no. 11 (2001): 2476–2483
AY Fadeev and TJ McCarthy “Self-Assembly Is Not the Only Reaction Possible Between Alkyltrichlorosilanes and Surfaces: Monomeric and Oligomeric Covalently Attached Layers of Dichloro- and Trichloroalkylsilanes on Silicon,” Langmuir 16, no. 18 (2000): 7268–7274
V Kumar et al “Fluorocarbon Coatings via Plasma Enhanced Chemical Vapor Deposition of 1H, 1H, 2H, 2H- Perfluorodecyl Acrylate -2, Morphology, Wettability and Antifouling Characterization,” Plasma Processes and Polymers 7 (2010): 926–938
S Kane “Surface Modification of Ultrahigh Molecular Weight Polyethylene to Improve Lubrication in Total Hip Replacements,” University of California, San Francisco with the University of California, Berkeley, 2008
CW Chang and D Sretavan, “Novel High-Resolution Micropatterning for Neuron Culture Using Polylysine Adsorption on a Cell Repellant, Plasma-Polymerized Background,” Langmuir 24 (2008): 13048–13057.
 Khoren Sahagian is a materials scientist involved in process development and research for gas plasma technologies at Plasma Technology Systems (Belmont, CA). In 2008 he was awarded the R&D 100 award, given to the 100 most significant innovations of the year.
Khoren Sahagian is a materials scientist involved in process development and research for gas plasma technologies at Plasma Technology Systems (Belmont, CA). In 2008 he was awarded the R&D 100 award, given to the 100 most significant innovations of the year.
 Stephen Kaplan is the founder of Plasma Technology Systems and currently serves as a technology consultant for the company. He previously founded Plasma Science Inc., which pioneered the development of large chamber low pressure primary gas plasma systems for industry. Stephen is a polymer chemist with 50 years of experience in the plastics industry focused on interfacial and surface properties of polymers.
Stephen Kaplan is the founder of Plasma Technology Systems and currently serves as a technology consultant for the company. He previously founded Plasma Science Inc., which pioneered the development of large chamber low pressure primary gas plasma systems for industry. Stephen is a polymer chemist with 50 years of experience in the plastics industry focused on interfacial and surface properties of polymers.
 Mikki Larner is the vice president of sales and marketing for Plasma Technology Systems and has 13 years of experience in plasma surface modification. She is an active member in the Surfaces in Biomaterials Foundation, the Society for the Advancement of Materials and Process Engineering, and the Society of Plastics Engineers.
Mikki Larner is the vice president of sales and marketing for Plasma Technology Systems and has 13 years of experience in plasma surface modification. She is an active member in the Surfaces in Biomaterials Foundation, the Society for the Advancement of Materials and Process Engineering, and the Society of Plastics Engineers.
About the Author(s)
You May Also Like