May 24, 2012
 Biomedical textiles
Biomedical textiles
Biomedical textiles supplied by Secant Medical feature complex fabric geometries and distinct fiber architectures that can enable biomedical device engineers to enhance their implantable device designs. Constructed from advanced polymeric, metallic, and resorbable biomaterials, these textiles offer design versatility with the right balance of fiber biomaterials, textile forming technologies, and unique fabric geometries to create a high-performing, minimally invasive device, according to the company. The properties of implantable textiles are engineered into products that require controlled porosity, expansion, compaction, radial and tensile strength, flexibility, absorbability, or shape-memory characteristics. Applications for these textile structures include endovascular stent grafts, endovascular aneurysm repair, suture devices, knotless fixation devices, soft-tissue anchors, hernia mesh, graft containment, fracture fixation, annulus repair, and tissue in-growth or regeneration for long-term implantation or resorption by the body.
Secant Medical
PERKASIE, PA
 Biomedical silicones
Biomedical silicones
The Elast-Eon carbonate silicone (ECSil) family of biomedical polymers, which offer the biological stability of the Elast-Eon polymers on which they are based, have been formulated by Aortech Polymers & Medical Devices to exhibit good physical properties across the range of five grades. Available in durometers of 70, 75, and 90 Shore A and 60 and 70 Shore D, these materials present a diversity of mechanical properties to cover a variety of medical applications. They are rated from 60 to 175 kN/m for tear strength, from 25 to 40 MPa for tensile strength, and from 8 to 450 MPa for modulus of elasticity. The softest grade offers an elongation at break of 750%, whereas the hardest has an elongation capacity of 300%. Supplied in pellet form, these silicone biomaterials have application in cardiac pacing leads, orthopedics, spinal disks, and other medical devices requiring high levels of mechanical performance.
AorTech Polymers & Medical Devices
Rogers, MN
Implantable polymer composite
Endolign composite from Invibio is an implantable polymer engineered to deliver strength and performance in high-load applications. The radiolucent, nonmetallic biomaterial is capable of replacing cobalt-chromium alloys, titanium alloys, and stainless steel in many applications that traditionally have employed these or other metals. It features good fatigue behavior, biocompatibility, and biostability and is supported by FDA drug and device master files. An inherently pure and inert composite made up of continuous carbon fibers in a PEEK-polymer matrix, the material is offered as an option for providing structural support or sustained or cyclic load-bearing capability in applications involving implantation or blood, bone, or tissue contact exceeding 30 days. Applications include such devices as translaminar fixation pins, spinal-fusion cages, bone fracture plates and screws, and intermedullary nails and screws.
Invibio Inc.
WEST CONSHOHOCKEN, PA
 Nitinol alloys and superalloys
Nitinol alloys and superalloys
A specialist in nitinol alloys and superalloys, Memry offers nitinol in semifinished tube, wire, sheet, and strip for interventional cardiology and radiology, surgical, orthopedic, urologic, and other applications. Nitinol is compatible with the human body and is able to accommodate large strains; it is suitable for both implantable and single-use medical devices. The manufacturer can supply drawn tubing, including small hypotubes, in a variety of finishes and sizes. Its flexible and kink-resistant superelastic nitinol wire supports complex component designs featuring intricate geometries. Sheet nitinol is also available for stamping, punching, and deep drawing while nitinol in strip form, which has all the properties and capabilities of wire, is supplied to dimensional and other specifications in cold-worked or fully superelastic states.
Memry
BETHEL, CT
 Polycarbonate urethanes
Polycarbonate urethanes
ChronoFlex AR and ChronoFlex AR-LT self-sealing polycarbonate urethanes are custom-designed for molding, casting, and dip-coating medical device applications. AdvanSource Biomaterials synthesizes these biocompatible materials fully in liquid to give them good strength and elongation properties while maintaining the inherent polycarbonate distinction of long-term durability and resistance to environmental stress cracking. The inherent low-tack property of the liquid polymers allows for pulsatile flow in situ. They may be electrospun or used in water emulsion processes. Offered in a range of configurations of viscosity and concentration and in antimicrobial forms, the materials are suited for applications requiring high flexural endurance, such as artificial heart valves and vascular grafts, and for use in fabricating blood-contacting surfaces, such as coatings.
AdvanSource Biomaterials
WILMINGTON, MA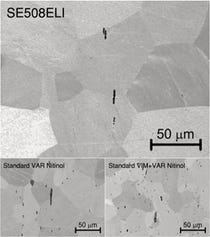
High-purity nitinol
An extra-low-interstitial (ELI) version of its implantable SE508 nitinol alloy, SE508ELI, is available from Nitinol Devices & Components Inc. for use in safety-critical medical device components. Owing to specially formulated raw materials and to melting techniques that minimize the formation of impurities through optimized solidification thermodynamics, this bulk nitinol alloy contains only very few and very small inclusions. It exhibits the same strength, ductility, transformation temperatures, superelasticity, formability, biocompatibility, and corrosion resistance as conventional nitinol, according to the company. In addition, it is virtually carbon free, precluding the presence of hard titanium carbide inclusions, and oxygen levels are nominally 60 ppm. The alloy can be supplied in the form of finished components or as raw material in the stock shapes of tube, wire, sheet, and strip.
Nitinol Devices & Components Inc.
FREMONT, CA Nonwoven biomedical textile
Nonwoven biomedical textile
A new version of the Biofelt absorbable scaffold for implantable devices used in orthopedic, cardiology, general surgery, and other in vivo applications is available from Biomedical Structures LLC, a specialist in the design, development, and manufacture of advanced biomedical textiles. The 3-D nonwoven, fibrous-matrix structure of the scaffold provides a platform that, with its large surface area and void volume, enables natural tissue in-growth in surgical applications. Produced from polyglycolic acid, poly-L lactic acid, and copolymers such as copolylactic acid-glycolic acid, the textile can serve as a bioabsorbable component of medical devices or surgical systems. It is custom-engineered to suit individual device requirements and offered with various densities and thicknesses. The scaffold material can be produced in flat sheets, disks, tubes, and other geometric shapes, with an absorption profile ranging from less than 30 days to one year.
Biomedical Structures LLC
WARWICK, RI
You May Also Like


