Originally Published MDDI May 2006 PLASTICSUnderstanding the structural and chemical properties of various types of silicones may give manufacturers a much-needed boost in choosing drug-delivery materials. Brian Reilly and Stephen Bruner
PLASTICS
|
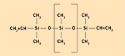
Figure 1. A typical polysiloxane structure, where R = CH3, phenyl (aromatic carbon ring), F3CCH2CH2, and CHCH2. |
Drug-delivery systems are some of the most rapidly proliferating products in healthcare today, with the implantable-systems market growing at a double-digit clip. As pharmaceutical companies look for additional patent protections and seek to improve patient compliance, novel delivery methods are now part of the product strategy.
Medical device industry manufacturers and their suppliers are in a unique position to leverage their expertise to provide delivery technologies. From system design to the materials used to build those systems, the medical device industry has already addressed many of the challenges the pharmaceutical industry faces as it develops drug-delivery systems.
Silicones, for example, are widely used materials in the medical device industry. These materials have a long history in medical devices and offer drug-delivery product engineers versatility and biocompatibility. A silicone system can also be tailored to fit a specific application. Already, manufacturers of drug-delivery devices are incorporating silicones in products that require a matrix, whereby the device is then capable of elution or ion release of an active additive or component. This article discusses the chemical properties of silicone systems, which are the key to silicone's flexibility and use in drug-delivery systems.
Healthcare Applications. Silicones expanded into healthcare and medical applications in the 1950s after extensive use in the aerospace industry in the previous decade. Within 20 years, a considerable body of work established that silicone oils and cross-linked siloxane systems did not give rise to harmful consequences when performing subcutaneous, intracutaneous, and intramuscular administrations. In 1954, J. D. B. McDougall reported the cultures of various tissues of warm-blooded animals known to be extraordinarily sensitive to foreign influences showed no deviation from the usual growth pattern on contact with liquid, semisolid, and rubberlike silicone products.1 Silicones have been characterized as biologically and toxicologically inert as a result of this work.2
Many applications, such as pacemaker leads, hydrocephalus shunts, heart valves, finger joints, and intraocular lenses, use silicone materials.
Commercial Drug-Delivery Applications. Commercial applications such as Norplant (Wyeth Pharmaceuticals; Madison, NJ) and Femring (Warner Chilcott; Rockaway, NJ) are examples of clinically successful drug-delivery applications that involve silicone materials. The patent cites a number of agents that could be used in drug-eluting applications. The drugs cited include antidepressants; anxiolytics; vitamins B6, D, and E; antifungals; opioid analgesics; nonopioid analgesics; and antiviral compounds.3
Polymer Chemistry
The chemistry behind silicone provides the material's versatility and enables it to be custom designed to fit particular drug-delivery applications. The versatile silicone polymer chemistry can provide varying properties useful for different applications. This polymer chemistry, when combined with reactive cross-linkers, catalysts, and reinforcing fillers, leads to a diverse set of material compositions.
The term silicone is actually a misnomer. Normally the suffix -one describes a substance that has a carbon atom double bonded to an oxygen atom in its backbone. Scientists believed initially that silicone materials contained double-bonded oxygen, hence the word silicone. However, silicones are really inorganic polymers having no carbon in the backbone and, therefore, they should be called polysiloxanes. Figure 1 shows their typical structure.
Because different constituents can be incorporated onto the polymer, polysiloxanes are used in a wide array of applications. Various polysiloxanes can provide a range of elastomeric properties that can be chosen according to a specific use. These types of silicones, or polysiloxanes, and their property advantages are presented below.
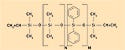
Dimethyl silicones, or dimethylpolysiloxanes, are the most common silicone polymers used industrially. These are typically the most cost-effective to produce and generally yield good physical properties in silicone elastomers and gels. The polymer pictured in Figure 2 contains vinyl end groups that participate in a platinum-catalyzed addition reaction (see the section on cure chemistry on page 104 for more information).
|
Figure 2. The chemical structure of a polymer that contains vinyl end groups that participate in a platinum-catalyzed addition reaction. |
Phenyl silicone systems contain diphenyldimethylpolysiloxane copolymers. The steric hindrance of the large phenyl groups significantly prohibits high concentrations of diphenyl units on the polymer chain. The phenyl functionality also boosts the refractive index of the polymers and silicone systems that use these polymers.
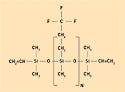
Silicone polymers with diphenyl functionality are used to biophotonic applications where higher-refractive-index materials can be used to create a thin lens (e.g., intraocular lenses). Devices with several layers of diphenyl elastomer systems may be created to control release rates of certain drugs. Figure 3 shows a typical structure for a methyl phenyl silicone.
|
Figure 3. A typical structure for a methyl phenyl silicone. |
Fluorosilicones are based on trifluoropropylmethylpolysiloxane polymers and are used for applications that require fuel or hydrocarbon resistance. The trifluoropropyl group contributes a slight polarity to the polymer, resulting in swell resistance to gasoline and jet fuels. However, polar solvents—such as methyl ethyl ketone and methyl isobutyl ketone—may significantly affect fluorosilicones. Although some fluorosilicones contain 100% trifluoropropylmethylpolysiloxane repeating units, other systems contain a combination of the fluorosiloxane units and dimethyl units to form a copolymer.
Adjusting the amount of trifluoropropylmethylsiloxane units in the polymerization phase provides optimal performance in specific applications. Figure 4 shows a typical structure for a fluorosilicone.
|
Figure 4. A typical structure for a fluorosilicone. |
Material Composition
Silicone is available in a broad range of material compositions, making it a viable option for many healthcare and drug-delivery applications. Some silicone material compositions and their typical applications are described below.
Silicone fluids are nonreactive silicone polymers formulated with dimethyl, methylphenyl, diphenyl, or trifluoropropylmethyl constituent groups. Their viscosities depend largely on the molecular weight of the polymer and on the steric hindrance of functional groups on the polymer chain. Fluids are typically used in lubrication and dampening applications.
Silicone gels contain reactive silicone polymers and reactive silicone cross-linkers. These materials are designed to have a soft, compliant feel when cured. Typical applications include tissue simulation and dampening.
Silicone pressure-sensitive adhesives (PSAs) are made from polymers and resins. Designed to perform in a cured or uncured state, PSAs form a nonpermanent bond with substrates such as metals, plastics, glass, and skin.
Silicone elastomers fall into several categories: high-consistency elastomers, liquid-silicone rubbers, low-consistency silicones, and adhesives.
High-consistency elastomers typically contain high-viscosity polymers and high levels of reinforcing silica. In their uncured state, these materials are claylike in consistency and offer good physical properties when vulcanized. High-consistency materials can be molded into parts by compression or transfer molding, or are more commonly used for extrusion to yield tubing configurations.
Liquid-silicone rubbers (LSRs) are elastomers containing medium-viscosity polymers and moderate amounts of silica. Once cured, the elastomers have good physical properties. Uncured, the consistency resembles that of petroleum jelly. These materials can be molded into parts and require the use of liquid-injection molding equipment.
Low-consistency silicones are pourable systems comprised of lower-viscosity polymers and reinforcing fillers such as silica or resin. These systems have lower physical properties than high-consistency elastomers or LSR formulations, but they can be easily processed and molded by manual methods. Low-consistency silicones can be molded into parts by compression molding or can be used as cured-in-place seals or gaskets.
Adhesives are low-consistency elastomers that contain lower-viscosity polymers, reinforcing silica, and adhesion promoters. Silicone adhesives are designed to adhere silicones to various substrate surfaces, including metals, glass, and certain plastics.
Cure Chemistry. When a manufacturer in the drug-delivery industry chooses a material for a specific application, material properties are not the only deciding factor. Manufacturers must also examine how the material will be used in a production setting. Processing limitations or vulcanization and curing by-products can render a chosen material ineffective for a specific application.
Silicones, however, can be designed around various cure chemistries to accommodate different production needs. Silicone systems can cure by platinum-catalyzed addition cure systems, tin-condensation cure systems, peroxide-cure systems, or oxime-cure systems. Some of the oldest cure chemistries used with silicones employ an acetoxy, tin-condensation cure system. These systems yield acetic acid, a by-product of the reaction.
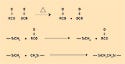
Platinum-catalyzed silicones use a platinum complex to participate in a chemical reaction between a hydride-functional siloxane polymer and a vinyl-functional siloxane polymer.
The result is an ethyl bridge between the two polymers (see Figure 5).
|
Figure 5. Platinum-catalyzed silicones create an ethyl bridge between a hydride-functional siloxane polymer and a vinyl-functional siloxane polymer. |
Platinum systems are typically cured quickly with heat, but can be formulated to cure at low temperatures or even at room temperature, if necessary. These systems offer a fast cure and no volatile by-products. However, their cure can also be inhibited (defined as either temporarily or permanently preventing system cross-linking). Some types of inhibitors are purposely added to these systems to control cure rate. But contact with tin, sulfur, and some amine-containing compounds may permanently inhibit cure. Compounds that inhibit cure can be identified easily by attempting to cure a platinum-catalyzed system in contact with the compound. Inhibition can be observed as uncatalyzed regions in the elastomer systems or as inconsistency in cure over time.
Tin-condensation systems involve hydroxyl-functional polymers and alkoxy-functional cross-linking compounds. The alkoxy-functional cross-linker first undergoes a hydrolysis step and yields a hydroxyl group. This hydroxyl group then participates in a condensation reaction with another hydroxyl group attached to the polymer. The reaction can proceed without the assistance of the tin catalyst, but the presence of the catalyst accelerates the rate of reaction. The reaction mechanism is depicted in Figure 6.
|
Figure 6. The reaction mechanism of a tin-condensation system. |
Condensation systems can cure at room temperature (which is useful for temperature-sensitive additives). They also provide robust cure systems that are difficult to inhibit. One disadvantage of condensation systems is the long cure time, often requiring hours or days for complete elastomer cure.
Peroxide-catalyzed systems, used mostly in high-consistency elastomers, have a reaction mechanism that involves a peroxide catalyst and either methyl groups or vinyl functional groups. The peroxide catalysts create free radical species of the methyl and vinyl that can then form covalent bonds. Figure 7 shows the reaction mechanism involving a peroxide catalysis of two methyl groups.
|
Figure 7. The reaction mechanism involving a peroxide catalysis of two methyl groups. |
Peroxide systems are typically robust (or not easily inhibited) and offer properties that are good for balloon applications, such as low tension set. Disadvantages include a lengthy, high- temperature postcuring step to remove the reaction's by-products. Other disadvantages include the possibility of the catalyst interacting with incorporated active agents.
Structure and Applications
For the purposes of this article, the drug-delivery systems discussed use the permeation characteristics of silicones in the delivery of pharmaceutical agents. Device configurations can range from reservoir designs to devices that deliver drugs from an elastomeric matrix. Both designs are cited in transdermal, transmucosal, and implanted drug-delivery designs. The key to these designs is the permeation of drugs and, specifically, the rate at which the agent permeates. Permeability of active agents is dependent on two key factors: solubility and diffusivity.
Solubility. The siloxane polymer backbone of repeating silicon and oxygen atoms creates a potential for interaction. The two free pairs of electrons associated with each oxygen atom can form hydrogen bonds with proton donors. Silicone elastomer systems can be strengthened with silica or resin reinforcement, which form hydrogen bonds with the polymer backbone.
|
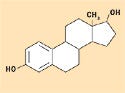
Figure 8. Estradiol. |
|
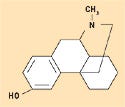
Figure 9. Levorphanol. |
|
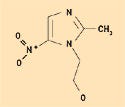
Figure 10. Metrondiazole. |
|
Figure 11. The chemical structure of linoleic acid. |
Despite its ability to form hydrogen bonds, silicone is considered hydrophobic in nature. The methyl constituency on the siloxane polymer backbone creates this effect, which is ideal for the solubility of lipophilic pharmaceutical agents having mostly nonpolar structures, with few alcohol or ketone groups. Figures 8–10 depict the molecular structures for estradiol, levorphanol, and metrondiazole.
Work in this area has determined that the melting point of a molecule, or the energy of disassociation, can be used to determine the solubility of similar molecules in silicone materials.4 Other studies suggest that the addition of polar groups onto an active agent can negatively affect the solubility of the agent in the silicone and, in turn, affect the permeability of that agent.2
It appears that the interaction between the oxygen of the siloxane backbone and the pharmaceutical agents does have some hydrogen bonding with the alcohol functionality of many active pharmaceutical agents. This is evidenced by a rise in release rates when a fatty acid ester is incorporated into the elastomer system. The molecular structure of linoleic acid is shown in Figure 11.
It is believed that fatty acid esters increase the hydrophobicity of the siloxane system.1 It can be speculated that the carboxylic acid group competes for siloxane oxygen, effectively reducing the concentration of siloxane oxygen available in the elastomer system.
The silicone polymer backbone can also be modified to improve the solubility of certain agents. Trifluoropropylmethylpolysiloxanes exhibit a slightly more polar characteristic and may improve the solubility of more-hydrophilic active agents.2 Conversely, because the large phenyl groups provide steric hindrance of the oxygen on the siloxane backbone, diphenyl functional siloxanes may improve the solubility of more-hydrophobic agents. Keep in mind that these modifications to the polymer may affect the second critical factor to drug permeability: diffusivity.
Diffusivity. The large atomic volume of the silicon atom, as well as the size and position of constituent groups, explains the virtually complete freedom of rotation around the Si-O-Si bond. Silicone polymers form helixes, and the bond angles of the silicon-oxygen bonds create large amounts of free volume in silicone elastomers. This free volume, and the high compressibility found in silicones, is associated with their permeability to gases and liquids. The gas permeability of silicone rubber is up to 100 times greater than that of natural or butyl rubber. Silicone rubbers swell in aliphatic, aromatic, and chlorinated hydrocarbon solvents.
Silicone gaskets for industrial applications absorb lubricating oils and tend to wet the surface of the elastomer system after the source of the lubrication is removed.5 This phenomenon enables polymer manufacturers to develop self-lubricating elastomer formulations. Proprietary silicone fluids are incorporated into the elastomer formulation and migrate to the surface of the molded component after cure.
The prevailing theory in this area is that the amount of free volume in an elastomer system and the relative freedom of movement of the polymer chains is the prime determinant in the diffusivity of an active agent. Work in this area relates molecular weight and molecular volume to diffusivity rates (i.e., the larger the molecule, the less diffusivity and, consequently, lower permeation rates).4 This work is supported by a separate study measuring average mesh size (a function of cross-link density) and diffusivity.6 In summary, it appears that the size of the molecule, the amount of space that molecule occupies, and the relative freedom with which the molecule moves from space to space affects diffusivity and, ultimately, permeability.
When developing silicone-based drug-delivery systems, solubility and diffusivity—the two factors critical to permeability—must be understood to determine whether the active agent and the silicone can produce the desired result. Should developers determine that the permeability of the silicone agent is ideal, further modifications to the silicone system may produce optimal release rates.
Drug-Delivery Applications
Evaluation and Fabrication. The first step in determining general compatibility of a silicone with an active agent is determining the agent's solubility in silicone. Silicone oil can be used to determine whether an agent may be soluble in a silicone elastomer system.5
Once solubility has been determined, the active agent can then be tested in the elastomer system to establish the optimal concentration or agent configuration for the target release rate per day and the total number of release days. In some devices, the drug is incorporated into a silicone matrix core, or reservoir, and the outer layer of silicone (without pharmaceutical agents incorporated) controls the release on the device. Such information can be found on patent registration forms.6-8
A general review suggests that 5–50% of the active agent is optimal for release rates of 10 to 500 µm of drug per day. These numbers are highly dependent on the type of drug and silicone, as well as any rate-enhancing additives. The release rate is also cited on patents and has been characterized as essentially zero order.
Conclusion
It is essential that device makers looking for delivery materials understand the chemical and structural properties of silicones. The chemistry of silicone and silicone materials and their interactive characteristics indicate the suitability of these materials for drug-delivery devices.
These materials are already used extensively in the healthcare industry and in existing drug-delivery applications. Evaluating these factors shows the versatility of silicone and how this versatility can benefit drug delivery. The interactions between drugs, release-enhancing agents, and silicone systems are all important factors when considering silicone materials.
References
1. K Malcolm et al., “Influence of Silicone Elastomer Solubility and Diffusivity on the In Vitro Release of Drugs from Intravaginal Rings,” Journal of Controlled Release 90, no. 2 (2003): 217–225.
2. M Ghannam, K Tojo, and Y Chien, Kinetics and Thermodynamics of Drug Permeation Through Silicone Elastomers (I) Effect of Penetrant Hydrophilicity (New York: Marcel Dekker, 1986): 303–325.
3. S Nabahi. Intravaginal drug-delivery device. U.S. Patent 6,039,968, filed June 22, 1998, and issued Mar. 21, 2000.
4. S Nabahi. Intravaginal drug-delivery device. U.S. Patent 5,788,980, filed Oct. 24, 1996, and issued Aug. 4, 1998.
5. W Noll, Chemistry and Technology of Silicones (New York: Academic Press, 1968).
6. A McClay. Intravaginal drug-delivery devices for the administration of 17.beta.-oestradiol precursors. U.S. Patent 5,855,906, filed June 3, 1997, and issued Jan. 5, 1999.
7. C Passmore et al. Intravaginal drug-delivery devices for the administration of testosterone and testosterone precursors. U.S. Patent 6,416,780, filed May 1, 2000, and issued July 9, 2002.
8. S Nabahi. Intravaginal drug-delivery device. U.S. Patent 6,103,256, filed Dec. 2, 1999, and issued Aug. 15, 2000.
Brian Reilly works in the R&D department at NuSil Technology LLC (Carpinteria, CA). Stephen Bruner is marketing director at NuSil and can be contacted at [email protected].
Copyright ©2006 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like