Many device design engineers and manufacturers do not realize the significant role that cleaning and coating methods can play in the consistency and quality of device performance once it’s in a doctor’s hands. Although it is sometimes an afterthought, cleaning and coating should be considered in every step of device design and manufacture.
May 5, 2010
Many device design engineers and manufacturers do not realize the significant role that cleaning and coating methods can play in the consistency and quality of device performance once it’s in a doctor’s hands. Although it is sometimes an afterthought, cleaning and coating should be considered in every step of device design and manufacture. As an added benefit, considering these challenges in advance will ensure more flexibility in the design and manufacturing process and will ultimately support maximum profitability through low capital costs.
|
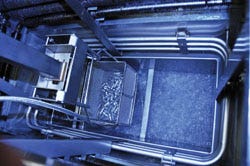
A vapor degreaser is part of the cleaning process for medical device cleaning and coating, especially in high volume. |
This article discusses the top cleaning and coating challenges that design engineers and manufacturers face during the process of bringing a device to production. It also outlines solutions and offers best practices to improve the cleaning and coating process (see the sidebar, “User Tips for Cleaning and Coating Professionals”).
Assessing Options
Engineers and manufacturers ultimately use cleaning and coating systems to get the best and most consistent performance out of a device. These systems also ensure that the device is sterile and ready for use once it reaches a physician.
When manufacturing medical devices, engineers must decide whether they must clean component parts needed for assembly, clean the final assembled product, or both. Virtually all devices will require some measure of cleaning to remove particulates, oils, or inorganic contamination that results from the manufacturing process. The challenge is to specify a cleaning process that is suitable for a variety of materials and geometries, including delicate plastic injection-molded parts, stainless-steel microtubing, or sophisticated implantable devices. Devices that may not need either cleaning or coating include silicone tubing, injection-molded silicone masks, or bulbs. For example, some silicone-molded parts may come directly from a molding machine and automatically move toward assembly and packaging, bypassing the cleaning step.
When it comes to the coating process (lubrication), not all devices will require a coating. Application of a lubricant coating is generally dictated by the desired performance of the medical device once assembled. A coating is typically applied to a device that will function better with reduced friction. Any device that slides, moves side to side, or rotates may be a candidate for a lubricant coating. In addition, devices such as a syringe needle or cannula for injecting medicine or fluids may be cleaned and then coated with silicone to reduce friction when the needle pierces the skin. Likewise, mechanical assemblies that consist of multiple component parts, such as a surgical stapler, often need a lubricant coating to reduce friction and address stacked tolerances.
Once the engineer or manufacturer identifies a device that must be cleaned or coated, the exact process is determined mainly by the required manufacturing volume. In low-volume production environments, basic cleaning devices such as aerosols, dry wipes, presaturated wipes with water-based cleaners, solvents, or solvent- and water-based cleaners may be acceptable. In high-volume production systems, the engineer will typically seek more automated cleaning systems to reduce costs and improve cleaning consistency. Those systems may be either solvent or water based and use machines engineered for the application.
Lubricant coatings applied in-house will typically be either silicone based or polytetrafluoroethylene (PTFE) based. Sophisticated surface treatment lubricants must be applied off the manufacturing premises, because they require advanced application methods to impart hydrophilic properties to surfaces that become lubricious when wetted with body fluids.
The key factors to consider when choosing a cleaning or coating system include worker safety, equipment costs, cost per part treated, materials compatibility, required floor space, FDA approvals or ease of obtaining approvals, reduced bioburden, and ease of use. Each application will have its own unique constraints, and the best thing to do when determining the cleaning and coating system is for the design and manufacturing engineer to meet with the cleaning and coating provider to discuss specific concerns.
|
This image shows a vapor degreaser with a basket of aluminum fluid dispenser nozzle assemblies, boiling solvent, and vapor cooling coils. |
Typically, the largest consideration in the cleaning process is cost-effectiveness (expressed as cost per part cleaned). Concerns prioritized after that are materials compatibility (i.e., can the fluid be used on plastic parts?), ease of use, and safety and environmental concerns.
For coating and lubrication, performance is usually the number one consideration. When the device has been manufactured and is in the hands of the physician, will it perform the way in which it was designed? Following this matter (as with the cleaning process), manufacturers and engineers are most concerned with materials’ compatibility, cost, and safety and environmental issues.
Engineers and manufacturers will also consider the class of the device when determining the desired coating and cleaning process. Each of the three classes of medical devices determined by FDA has a different level of control necessary to ensure the safety and effectiveness of the device. Class I medical devices present minimal potential for harm to the user and are often simpler in design than Class II or III devices. They include tongue depressors, bedpans, exam gloves, and handheld surgical instruments. Class II devices are subject to more regulation because they present a bigger potential safety risk to the patient but are still typically noninvasive. These devices include x-ray machines, infusion pumps, surgical drapes, needles, and sutures. Class III devices are the most highly regulated and are life supporting or sustaining. They include heart valves, cerebral stimulators, pacemakers, and other implantable devices.
The type of cleaning and coating process chosen is dependent on whether the device will be invasive. For example, silicone coatings are often used on invasive devices due to their compatibility and safety in human tissues; PTFE coatings are often used on mechanical assemblies, such as surgical staplers, that function outside of the body.
When considering cleaning and coating options, it is important to partner with a cleaning and coatings provider that understands EPA regulations and the chemistry involved in the process. The device engineer is typically the expert in FDA regulation. However, the cleaning and coating partner should provide a dimension of expertise on EPA regulations and chemistry parameters to make the design and manufacture of the device as consistent, efficient, and sustainable as possible. Ultimately, this can save the manufacturer and designer time and money.
The Challenges
Cosmetics. Device engineers and manufacturers must be concerned with the cosmetic appearance of the device because they need the doctor, nurse, or patient to see a device with surfaces that are smooth and clean. The optimal surface is obtained through cleaning and coating processes that are used to eliminate cosmetic defects such as fingerprints or particulates that remain from the manufacturing process.
When coating a device with a lubricant such as silicone, which can leave an oily finish, it may be necessary to remove the coating from exposed surfaces so the device is visually perfect. With other coatings, such as dry lubricants, the coating dries completely and uniformly once dipped or sprayed.
Bioburden. Bioburden is a challenge in the packaging, storing, and sterilization of devices after cleaning and coating. It is quite common with manufacturers that use aqueous cleaners and often occurs when a device is not completely dry when it is packaged.
Technically, bioburden is a measure of an object’s contamination with microorganisms. The presence of microorganisms on a medical device can also lead to the presence of endotoxins. Both can present challenges in delivering a sterile and pyrogen-free medical device to the end-user.
Many conditions can cause bioburden but fundamentally, water is a growth medium for bacteria. Therefore, removing water from the cleaning or coating process removes a growth medium for bioburden. This is why solvents are often preferable to aqueous (water-based) cleaners or coatings—because they present an environment that is hostile to bacteria growth.
If bioburden is not addressed, it can lead to infection when that device is used on a patient. An engineer or manufacturer who is addressing bioburden can specify a solvent-based process with submicron filtration.
Stacked Tolerances. One of the most familiar challenges that medical device design engineers and manufacturers face is stacked tolerances in mechanical assemblies, which can create user challenges for device actuation. This is a particularly common challenge with complex, single-use mechanical assemblies such as staplers and arthroscopic devices.
In engineering, the tolerance refers to the permissible limit of variation in a physical dimension. Tolerances are specified by the design engineer to allow reasonable leeway for imperfections and variability without compromising performance. Tolerances become a challenge for design engineers and manufacturers when they begin to stack up against each other. For example, when a mechanical assembly such as a medical stapler is assembled, the tolerances of each metal stamping, spring, or plastic part may begin to combine in such a way that the assembled device requires more force to actuate or execute. This issue is most common in high-volume production, when tooling used to manufacture metal stampings, springs, and plastic parts begins to wear.
|
A partially assembled medical device consists of metal and plastic rails and a spring, which slide against each other, and is treated with a Duraglide coating. |
There are several ways that design engineers and manufacturers can deal with stacking tolerances. Engineers can choose to design everything with tighter tolerances to gain high precision. However, precision commonly leads to more frequent inspection and maintenance of tooling and fixtures during manufacturing, which drives up the unit price of a finished device. A more common way of dealing with stacked tolerances is to apply a lubricant coating such as PTFE or silicone on the finished assembly to reduce friction.
Dry lubricants using PTFE particles are typically the best way for the design engineer and manufacturer to reduce the effect of tolerance stacking. In fact, many single-use medical devices that are on the market would not be commercially viable without this coating. Dry lubricants are used on many devices or mechanical assemblies found in the operating room, including catheters, cutting tools, staplers, hypotubes, and other surface-to-surface complex assemblies.
Dry lubricants reduce the force needed to actuate or execute a device by 25–30% and provide a silky, almost effortless actuation for the medical professional performing the procedure. In comparison to silicone coatings, which are oil based, dry lubricants impart a lower coefficient of friction and are nonmigrating, so they will not transfer to packaging.
Maintaining Calibration. Maintaining calibration of lubricant dispersions and fluids is important to the consistency and quality of the coating and thus the performance of the device. For dry lubricants in particular, this is a top challenge for device manufacturers.
The first challenge to maintaining calibration is controlling the evaporation of carrier fluid. Many PTFE dry lubricants are mixed with a carrier fluid that evaporates very quickly. This is necessary and good for the coating of the part because it dries quickly enough to leave a very consistent coating on the device. However, this also means that during the coating process, the fluid can evaporate quickly out of the vessel used for coating. In some cases, manufacturers will add an unmeasured amount of carrier fluid to maintain approximate percentage saturation, but this is not precise and can affect the quality of the coating.
To control evaporation and keep fluids calibrated for maximum consistency and quality, use of process-specific equipment for the cleaning and coating process is highly recommended. This may include hermetically sealed equipment, specialized solvent recovery systems, controlled temperature baths, engineered parts feeding systems such as hoists or conveyers, or engineered application systems such as spray or brush applicators.
Another challenge in fluid calibration is the PTFE particles themselves. Many coatings with PTFE micropowders require constant agitation because the particles have low hang time in the liquid carrier. Low hang time means that as the fluid sits in the vessel and parts are dipped, more of the PTFE particles will sink in the fluid to the bottom of the vessel. Many manufacturers address this issue by constantly agitating the fluid. However, if done improperly, this practice can also be inconsistent and lead to streaky coatings.
A key solution to the short hang time of PTFE particles in a coating application is to find a provider that uses a precalibrated fluid with micropowders. For example, MicroCare Medical’s Duraglide dry film lubricants use a premixed and calibrated formula that maintains the ratio of carrier fluid to PTFE particles. In addition, a proprietary microdispersion PTFE technology deposits a thin, smooth film over the treated surface. These microdispersions suspend the PTFE in unique carrier fluids to create a more effective hang time, which results in a consistent coating and smooth device movements.
Hang time of PTFE particles in the lubricant process can also be controlled or enhanced through the use of heat (i.e., maintaining a rolling boil of the fluid), ultrasonics, closed-loop circulation systems, and mechanical agitation. Engineers and manufacturers should also take care in choosing a method, because any of these methods to maintain agitation can also cause accelerated evaporation if not properly instituted into the process.
Facilities. There are several facility challenges that manufacturers in particular face in the process of bringing the device to production. One concern is the machine footprint when selecting a cleaning and coating process. For example, how much space does the equipment need, and what effect does this have on other facility costs or incidentals? Depending on the selected cleaning system, there will be different requirements for space, electricity, maintenance, and access to water systems.
In general, water-based systems have a bigger footprint than solvent-based systems. There is a wide variety and variation in the types of systems used to clean and coat devices, so it is not fair to say that one system is the most effective for reducing footprint. As a result, it is important to work closely with cleanings and coatings providers that can engineer to specific manufacturing needs.
Timing. Another concern for manufacturers is defining the point in the process at which they should clean and then coat the devices with lubricant. This timing affects the entire manufacturing and device delivery process. A lubricant coating is sometimes applied during the manufacturing process to assist in the assembly of the device. In other cases, it is applied at the end of the manufacturing process to ensure the ease of operation. The coating can also be applied both during and after assembly.
Parts should always be cleaned before applying lubrication to be certain that the coating will properly adhere to the treated surface. Lubricant does not properly adhere to a contaminated surface. The timing of cleaning and coating a device is determined on an individual basis. The cleaning or coating partner can advise the engineer and manufacturer on the appropriate timing for the specific device and system.
Environmental Concerns. Environmental regulations create new challenges for coating and lubrication, and this is a top concern for both manufacturers and engineers. All parties want to deliver the most effective cleaning and coating with the least environmental impact. Given the different options for cleaning and coating, there are obviously products that will have lower environmental impact than others, but this also must be weighed against all types of waste. For example, water, which is natural, can be used to clean devices, but the overall environmental impact of that system may be greater than a solvent system after considering the footprint of the system, the electricity used, the maintenance required, controlling bioburden, and the amount of water that is actually used. All factors must be weighed in determining the environmental impact—not just the product itself.
One factor that can reduce waste and increase sustainability is the use of engineered (versus manual) systems for cleaning and coating applications. When the process is automated, the user gains efficiencies that not only lead to consistency, but also lead to less waste.
Conclusion
Overall, it is important to work with a competent cleaning and coating provider as a partner. Its experience with all solvents and equipment should help the manufacturer and engineer choose the best process for a specific application.
An important fact for engineers and manufacturers to remember when designing medical devices and assemblies is that the cleaning and coating process can play a huge role in the performance, quality, and consistency of the finished device. Although there are plenty of challenges that can affect the process, the ultimate best practice is to find an expert cleaning and coating provider that can address any questions and concerns in a timely and educated manner. Successfully working with a partner will not only increase the quality and consistency of the process and performance of the device, but the manufacturer will also realize flexibility in the design and manufacturing process as well as achieve maximum profitability.
Jay Tourigny is vice president of operations at MicroCare Medical (New Britain, CT).
About the Author(s)
You May Also Like